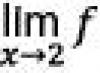Potassium sodium tartrate- acidity regulator, acidifier, antioxidant synergist, salt substitute, emulsifying salt, color stabilizer.
Potassium sodium tartrate physical and chemical properties
Colorless transparent crystals, white in bulk, have a salty, tongue-cooling taste. Soluble in water (1 g in 1 ml); insoluble in ethanol, oils, fats.
Potassium sodium tartrate natural source
In many types of vegetables and fruits in the form of tartaric acid.
Preparation of potassium sodium tartrate
The interaction of L-tartaric acid with sodium hydroxide and potassium hydroxide or sodium and potassium carbonates. Impurities: malates, other tarts, oxalates. According to tartaric acid content.
Daily intake of potassium sodium tartrate
ADI 30 mg/kg body weight per day in terms of L(+)-tartaric acid. There are no dangers according to GN-98.
Mention in standards and application in the food industry E-337
E-337 approved as an acidity regulator for the following food products: soups, broths in amounts up to 250 mg/kg of finished product; jams, preserves, jellies, citrus marmalades up to 3 g/kg individually or in combination with TARTARIC ACID, FUMARIC ACID and its salts to maintain pH between 2.8 and 3.5; GMP margarines. In the Russian Federation, it is allowed in canned fruits and vegetables in quantities according to TI (clause 3.1.18 of SanPiN 2.3.2.1293-03); in wines, drinks, food concentrates and other products, in bakery and flour confectionery products in quantities according to TI individually or in combination with tartaric acid and tartrates (clause 3.2.3,3.6.52 SanPiN 2.3.2.1293-03). Tartrates form stable complexes with iron and heavy metals and thus act as antioxidant synergists. They are sometimes used as melting salts in the production of processed cheese. The slow release of potassium and calcium from their salts regulates the rate of gelation of carrageenan, alginate and pectin gels. Sodium potassium tartrate can be used as a salt substitute.
Other uses of potassium sodium tartrate:
E-337 used in the production of pharmaceuticals, as well as for silvering mirrors, as a mouthwash, laxative
- an organic compound, crystalline hydrate of double (potassium and sodium) salt of tartaric acid. This substance was named Rochelle salt in honor of a pharmacist from France, who in the 17th century made it from winemaking waste and used it as a laxative. Formula - KNaC4H4O6.4H2O. "Rochelle salt" is the traditional name. Chemists use: potassium-sodium tartrate 4-water or potassium-sodium tartrate tetrahydrate.
Rochelle salt is obtained artificially by a chemical reaction of tartaric acid with potassium carbonate (carbonate) and sodium.
Properties
Under normal conditions, it is a crystalline substance, salty in taste, with a cooling effect. Crystals can be colorless, white, or bluish.
They have a complex structure and shape, do not have a center of symmetry, and are twelve-sided. They dissolve well in water, do not dissolve in alcohol. The reagent begins to decompose already at temperatures above +56 °C. When strongly heated, it first loses water of crystallization, then decomposes into potassium-sodium carbonate, water and carbon monoxide. It has ferroelectric and piezoelectric properties. Rochelle salt is non-toxic, non-flammable, fire- and explosion-proof.
Rochelle salt crystals were the first to have special electrical properties discovered at the end of the 19th century—spontaneous polarization in a certain temperature range. It turned out that the polarization of the crystal can be controlled by a strong magnetic field. After this, the entire class of substances with similar properties began to be called ferroelectrics. Today, more than seven hundred substances belong to this class.
Sodium potassium tartrate crystals aroused even greater interest when Pierre and Jean Curie discovered a piezoelectric effect in them, three thousand times stronger than that of quartz crystals. The piezoelectric effect is the occurrence of electrical discharges under mechanical action on a solid body. On its basis, before World War II, many different electronic devices were developed: ultrasonic locators, loudspeakers, hearing aids, medical probes, telephones, microphones and many others. Most were originally designed for wafers cut from quartz crystals, but these were very expensive and required sophisticated cutting equipment with diamond saws. Rochelle salt crystals were much cheaper and could simply be cut with damp thread. Unfortunately, they were fragile, unstable to temperature changes and high humidity. In the end, these shortcomings were overcome by placing the plates in waterproof shells. Mass production of electronic piezoelectric devices based on the reagent began. As the famous academician Alexandrov said: “Rochelle salt turned out to be a gold mine for physicists.”
The production of devices and devices based on the piezoelectric effect stimulated the creation of a new industry - the growing of large ferroelectric crystals. During the war, in the Soviet Union, it was possible to reduce the growing time for one and a half to two kilogram crystals of Rochelle salt from the original six months to 8-9 days. The army needed them so much that they were grown even in besieged Leningrad. In total, 54 tons of crystals were grown during the war. They were used in the manufacture of laryngophones (a microphone-type device that uses vibrations of the skin on the larynx) for tank crews and pilots, devices for underwater location and communications, piezoelectric fuses, loudspeakers from which the population received all news and information, etc.
Currently, Rochelle salt plates are almost never used as piezoelectrics. They were replaced by stronger crystals, for example, barium titanate.
Application 
In the chemical industry - in organic syntheses for the destruction of emulsions in aqueous solutions; in analytical chemistry for the preparation of buffer and standard solutions; is part of the reagents for aldehydes and ketones.
. In the food industry, as an antioxidant and leavening agent, an emulsifier in cheese making; included in baking powders.
. For silvering mirrors.
. In radio engineering and electronics.
. For cleaning gold-plated bronze (Rochelle salt does not react with copper oxides, so only copper salts and hydrates are removed).
. For the preparation of electrolytes for electroplating.
. In medicine, biology, and analytical chemistry, they are used to prepare solutions for the detection of sugars and proteins.
. To obtain medications, including laxatives in pharmacology.
. As a plant growth stimulator in agriculture.
Rochelle salt "analytical grade" (potassium-sodium tartrate, tartrate, potassium-sodium tartrate) is a tetrahydrate of sodium-potassium double salt of tartaric acid in the form of colorless or white hygroscopic crystals of the orthorhombic system. We offer to buy this product in our company.
The material is produced industrially by reacting tartaric acid with sodium and potassium carbonate salts. When producing using this method, it becomes possible to increase the size of the crystals if necessary. In laboratory practice, the reagent is synthesized by precipitation from a heated solution of potassium acid tartrate in fine-crystalline form by adding stoichiometric amounts of sodium carbonate.
Rochelle salt, which has a low price per kg, is characterized by weak alkalinity and increased hygroscopicity. It mixes well with water, but does not dissolve in ethyl alcohol. When heated to 55.6°C, the reagent begins to decompose. It has piezoelectric properties, which have led to its demand in technology: in telephone handsets, electrophone pickups, hearing aids, microphones and other similar devices.
Scope of application
Potassium sodium tartrate, the price of which in our company is favorable, is used as a demulsifier in aqueous solutions, as well as when carrying out reactions using aluminum hydride in organic synthesis processes. It also applies:
- as part of Feling liquid for the detection of sugars;
- for determination of proteins in solutions by the biuret method;
- as a reducing agent for silvering mirrors using the Heinrichson method;
- to increase the service life of cigarette paper in the manufacture of cigarettes;
- as an emulsifier in cheese making, antioxidant additive and leavening agent E337, as a component of baking powders in the food industry;
- as a gearbox in the printing industry;
- to increase the time interval before the plaster begins to harden in construction;
- as a component of Seydlitz powder in the production of laxatives, as well as the production of effervescent and instant preparations;
- to increase the saturation of the solution with anodes, create a good homogeneous surface and provide the ability to work at high concentrations as part of galvanic baths;
- when cleaning bronze with gilding, since the reagent does not react with copper oxides;
- as a plant growth stimulator in agriculture;
- as a desalting agent in the chemical industry.
This material is sold in our organization at an affordable price; you can buy it in 25 kg packaging.
Precautions
The product is non-toxic, does not burn, does not ignite and does not form explosive mixtures with air. In terms of the degree of impact on the body, it belongs to the group of substances of the third hazard class. When working with it, you must use protective equipment: plastic glasses, thick rubber gloves, an industrial respirator, special shoes and clothing. This material is sold in rubles. at AQUAHIM LLC.
Storage Features
The reagent should be stored in closed containers in dry, ventilated warehouses. Shelf life – 36 months from the date of manufacture.
Rochelle salt: where is it sold with delivery throughout the Russian Federation?
We sell chemical products at affordable prices. If you want to buy quality goods with shipping throughout Russia, we suggest that you familiarize yourself with the full price list of our online store on the main page of this site. To place an order, simply click on the green button above and enter your contact details in the field that opens. Our employee will call you back during business hours.
Physical properties and application in technology
In addition, potassium sodium tartrate tetrahydrate is one of the first substances that were discovered to have piezoelectric properties (Pierre and Jacques Curie, ). Later, these properties found application in technology: first in the interwar period in the USA (patent of the company BRUSH No. 2483647), and then in other countries (in the USSR in 2010), Rochelle salt began to be used in electric phone pickups, microphones, telephone handsets and other similar devices (for example, in hearing aids). This substance found application especially widely during times of increased demand for electrical equipment in the post-war years. Compared to other converters, the output voltage of Rochelle salt is very high (even three thousand times more). However, converters made from it cannot be stored in a humid place, since salt gradually melts due to its hygroscopicity.
Chemical properties and application
Sodium potassium tartrate is a component of Fehling's liquid, in which it is used to detect sugars. Rochelle salt is also used in silvering mirrors using the Heinrichson method. Additionally, this salt is used in organic synthesis as a demulsifier in aqueous solutions, usually in reactions involving aluminum hydride. Finally, the solution for the determination of proteins by the biuret method also contains sodium potassium tartrate.
Other uses
Potassium sodium tartrate is used in the food industry as an additive E337(antioxidant). It has a salty, cooling taste. This salt is also used in baking powders. The substance also found use in medicine - as a laxative (allegedly the pharmacist Seignet used this salt to help with stomach disorders). For these purposes, Rochelle salt is now often used as part of Seydlitz powder.
See also
Write a review about the article "Segnet salt"
Notes
An excerpt characterizing Rochelle salt
- Call, call. “Pitiful boy,” Denisov repeated.Petya was standing at the door when Denisov said this. Petya crawled between the officers and came close to Denisov.
“Let me kiss you, my dear,” he said. - Oh, how great! how good! - And, having kissed Denisov, he ran into the yard.
- Bosse! Vincent! – Petya shouted, stopping at the door.
- Who do you want, sir? - said a voice from the darkness. Petya answered that the boy was French, who was taken today.
- A! Spring? - said the Cossack.
His name Vincent has already been changed: the Cossacks - into Vesenny, and the men and soldiers - into Visenya. In both adaptations, this reminder of spring coincided with the idea of a young boy.
“He was warming himself by the fire there.” Hey Visenya! Visenya! Spring! – voices and laughter were heard in the darkness.
“And the boy is smart,” said the hussar standing next to Petya. “We fed him just now.” Passion was hungry!
Footsteps were heard in the darkness and, bare feet splashing in the mud, the drummer approached the door.
“Ah, c"est vous!" said Petya. “Voulez vous manger? N"ayez pas peur, on ne vous fera pas de mal,” he added, timidly and affectionately touching his hand. - Entrez, entrez. [Oh, it's you! Are you hungry? Don't be afraid, they won't do anything to you. Enter, enter.]
“Merci, monsieur, [Thank you, sir.],” answered the drummer in a trembling, almost childish voice and began to wipe his dirty feet on the threshold. Petya wanted to say a lot to the drummer, but he didn’t dare. He stood next to him in the hallway, shifting. Then in the darkness I took his hand and shook it.
“Entrez, entrez,” he repeated only in a gentle whisper.
“Oh, what should I do to him!” - Petya said to himself and, opening the door, let the boy pass by.
When the drummer entered the hut, Petya sat away from him, considering it humiliating for himself to pay attention to him. He just felt the money in his pocket and was in doubt whether it would be a shame to give it to the drummer.
From the drummer, who, on Denisov’s orders, was given vodka, lamb and whom Denisov ordered to dress in a Russian caftan, so that, without sending him away with the prisoners, he would be left with the party, Petya’s attention was diverted by Dolokhov’s arrival. Petya in the army heard many stories about the extraordinary courage and cruelty of Dolokhov with the French, and therefore, from the moment Dolokhov entered the hut, Petya, without taking his eyes off, looked at him and became more and more encouraged, twitching his head raised, so as not to be unworthy even of such a society as Dolokhov.
Dolokhov’s appearance strangely struck Petya with its simplicity.
Denisov dressed in a checkmen, wore a beard and on his chest the image of St. Nicholas the Wonderworker, and in his manner of speaking, in all his manners, he showed the peculiarity of his position. Dolokhov, on the contrary, previously in Moscow, who had worn a Persian suit, now had the appearance of the most prim Guards officer. His face was clean-shaven, he was dressed in a guards padded frock coat with George in the buttonhole and a simple cap straight on. He took off his wet cloak in the corner and, going up to Denisov, without greeting anyone, immediately began asking about the matter. Denisov told him about the plans that large detachments had for their transport, and about sending Petya, and about how he responded to both generals. Then Denisov told everything he knew about the position of the French detachment.
“That’s true, but you need to know what and how many troops,” said Dolokhov, “you will need to go.” Without knowing exactly how many there are, you cannot start the business. I like to do things carefully. Now, would any of the gentlemen want to go with me to their camp? I have my uniforms with me.
- I, I... I will go with you! – Petya screamed.
“You don’t need to go at all,” Denisov said, turning to Dolokhov, “and I won’t let him in for anything.”
- That's great! - Petya cried out, - why shouldn’t I go?..
- Yes, because there is no need.
“Well, excuse me, because... because... I’ll go, that’s all.” Will you take me? – he turned to Dolokhov.
“Why…” answered Dolokhov absentmindedly, peering into the face of the French drummer.
- How long have you had this young man? – he asked Denisov.
- Today they took him, but he doesn’t know anything. I left it for myself.
- Well, where are you putting the rest? - said Dolokhov.
- How to where? “I’m sending you under guard!” Denisov suddenly blushed and cried out. “And I’ll boldly say that I don’t have a single person on my conscience. Are you happy to send someone away? than magic, I will tell you, the honor of a soldier.
“It’s decent for a young count of sixteen to say these pleasantries,” Dolokhov said with a cold grin, “but it’s time for you to leave it.”
“Well, I’m not saying anything, I’m just saying that I will definitely go with you,” Petya said timidly.
“And it’s time for you and me, brother, to give up these pleasantries,” Dolokhov continued, as if he found special pleasure in talking about this subject that irritated Denisov. - Well, why did you take this to you? - he said, shaking his head. - Then why do you feel sorry for him? After all, we know these receipts of yours. You send them a hundred people, and thirty will come. They will starve or be beaten. So is it all the same not to take them?
Esaul, narrowing his bright eyes, nodded his head approvingly.
- This is all shit, there’s nothing to argue about. I don’t want to take it on my soul. You talk - help. Well, hog "osho." Just not from me.
Dolokhov laughed.
“Who didn’t tell them to catch me twenty times?” But they will catch me and you, with your chivalry, anyway. – He paused. - However, we need to do something. Send my Cossack with a pack! I have two French uniforms. Well, are you coming with me? – he asked Petya.
- I? Yes, yes, absolutely,” Petya cried, blushing almost to the point of tears, looking at Denisov.
Again, while Dolokhov was arguing with Denisov about what should be done with the prisoners, Petya felt awkward and hasty; but again I did not have time to fully understand what they were talking about. “If big, famous people think so, then it must be so, therefore it’s good,” he thought. “And most importantly, Denisov must not dare to think that I will obey him, that he can command me.” I will definitely go with Dolokhov to the French camp. He can do it and so can I.”
To all of Denisov’s urgings not to travel, Petya replied that he, too, was used to doing everything carefully, and not Lazar’s at random, and that he never thought about danger to himself.
“Because,” you yourself must agree, “if you don’t know correctly how many there are, the lives of maybe hundreds depend on it, but here we are alone, and then I really want this, and I will definitely, definitely go, you won’t stop me.” “, he said, “it will only get worse...
Dressed in French greatcoats and shakos, Petya and Dolokhov drove to the clearing from which Denisov looked at the camp, and, leaving the forest in complete darkness, descended into the ravine. Having driven down, Dolokhov ordered the Cossacks accompanying him to wait here and rode at a fast trot along the road to the bridge. Petya, transfixed with excitement, rode next to him.
“If we get caught, I won’t give up alive, I have a gun,” Petya whispered.
“Don’t speak Russian,” Dolokhov said in a quick whisper, and at that same moment a call was heard in the darkness: “Qui vive?” [Who's coming?] and the ringing of a gun.
Potassium-sodium tartrate GOST 5845-79
KNaC 4 H 4 O 6 4H 2 O
Rochelle salt- tetrahydrate of double sodium-potassium salt of tartaric acid ( sodium potassium tartrate). Named after the French pharmacist Pierre Seignet (fr. Pierre Seignette), 1660-1719 (other sources indicate the name of the pharmacist Elie Seigner (1632-1698), as well as the years of obtaining salt - 1672 and 1675).
Chemical properties and application
Since sodium potassium tartrate is a salt of tartaric acid, several optical isomers correspond to it. Only L-(+)-tartaric acid occurs in nature
The tetrahydrate is highly soluble in water (54 g/100 g) at 15 °C, at 30 °C 1390 g/l), and the salt is hygroscopic. However, the salt as such is obviously poorly soluble, since it precipitates during the preparation reaction.
Sodium potassium tartrate is a component of Fehling's liquid, in which it is used to detect sugars. Rochelle salt is also used in silvering mirrors using the Heinrichson method. In addition, this salt is used in organic synthesis as a demulsifier in aqueous solutions, usually in reactions involving aluminum hydride. Finally, the solution for the determination of proteins by the biuret method also contains sodium potassium tartrate.
In the laboratory, this salt is obtained by precipitation in fine crystalline form from a hot solution of potassium acid tartrate by adding a stoichiometric amount of Na 2 CO 3.