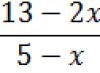In 1833, J. Berzelius coined the term “polymerism,” which he used to name one of the types of isomerism. Such substances (polymers) had to have the same composition, but different molecular weights, such as ethylene and butylene. The conclusion of J. Berzelius does not correspond to the modern understanding of the term “polymer”, because true (synthetic) polymers were not yet known at that time. The first mentions of synthetic polymers date back to 1838 (polyvinylidene chloride) and 1839 (polystyrene).
Polymer chemistry arose only after A. M. Butlerov created the theory of the chemical structure of organic compounds and was further developed thanks to an intensive search for methods of synthesizing rubber (G. Bushard, W. Tilden, K. Harries, I. L. Kondakov, S. V. Lebedev) . Since the beginning of the 20s of the 20th century, theoretical ideas about the structure of polymers began to develop.
DEFINITION
Polymers- chemical compounds with high molecular weight (from several thousand to many millions), the molecules of which (macromolecules) consist of a large number of repeating groups (monomer units).
Classification of polymers
The classification of polymers is based on three characteristics: their origin, chemical nature and differences in the main chain.
From the point of view of origin, all polymers are divided into natural (natural), which include nucleic acids, proteins, cellulose, natural rubber, amber; synthetic (obtained in the laboratory by synthesis and having no natural analogues), which include polyurethane, polyvinylidene fluoride, phenol-formaldehyde resins, etc.; artificial (obtained in the laboratory by synthesis, but based on natural polymers) - nitrocellulose, etc.
Based on their chemical nature, polymers are divided into organic polymers (based on a monomer - an organic substance - all synthetic polymers), inorganic (based on Si, Ge, S and other inorganic elements - polysilanes, polysilicic acids) and organoelement (a mixture of organic and inorganic polymers – polysoxanes) of nature.
There are homochain and heterochain polymers. In the first case, the main chain consists of carbon or silicon atoms (polysilanes, polystyrene), in the second - a skeleton of various atoms (polyamides, proteins).
Physical properties of polymers
Polymers are characterized by two states of aggregation - crystalline and amorphous - and special properties - elasticity (reversible deformations under light load - rubber), low fragility (plastics), orientation under the action of a directed mechanical field, high viscosity, and the dissolution of the polymer occurs through its swelling.
Preparation of polymers
Polymerization reactions are chain reactions that represent the sequential addition of molecules of unsaturated compounds to each other with the formation of a high-molecular-weight product - a polymer (Fig. 1).
Rice. 1. General scheme for polymer production
For example, polyethylene is produced by polymerization of ethylene. The molecular weight of the molecule reaches 1 million.
n CH 2 =CH 2 = -(-CH 2 -CH 2 -)-
Chemical properties of polymers
First of all, polymers will be characterized by reactions characteristic of the functional group present in the polymer. For example, if the polymer contains a hydroxo group characteristic of the class of alcohols, therefore, the polymer will participate in reactions like alcohols.
Secondly, interaction with low molecular weight compounds, interaction of polymers with each other with the formation of network or branched polymers, reactions between functional groups that are part of the same polymer, as well as the decomposition of the polymer into monomers (chain destruction).
Application of polymers
The production of polymers has found wide application in various areas of human life - the chemical industry (plastic production), machine and aircraft construction, oil refining enterprises, medicine and pharmacology, agriculture (production of herbicides, insecticides, pesticides), construction industry (sound and thermal insulation), production of toys, windows, pipes, household items.
Examples of problem solving
EXAMPLE 1
EXAMPLE 1
| Exercise | Polystyrene is highly soluble in non-polar organic solvents: benzene, toluene, xylene, carbon tetrachloride. Calculate the mass fraction (%) of polystyrene in a solution obtained by dissolving 25 g of polystyrene in benzene weighing 85 g. (22.73%). |
| Solution | We write down the formula for finding the mass fraction:
Let's find the mass of benzene solution: m solution (C 6 H 6) = m (C 6 H 6)/(/100%) |
There is practically no person in the modern world who does not have at least some idea about polymers. Polymers go through life with a person, making his life more and more convenient and comfortable. When mentioning polymers, the first associations will be with synthetic organic substances, since they are more visible. Natural polymers - natural organic substances - although there are more of them in the world around us, in the associative perception of a person they fade into the background. They always surround us, but no one thinks about the nature of the origin of flora and fauna. Cellulose, starch, lignin, rubber, proteins and nucleic acids are the main materials used by nature to create the animal and plant world around us. And absolutely no one will perceive precious stones, graphite, mica, sand and clay, glass and cement as polymers. Nevertheless, science has established the fact of the polymeric structure of many inorganic compounds, including those listed above. Polymer substances consist of macromolecules. When polymers are formed, a large number of atoms or groups of atoms are bonded to each other by chemical bonds - covalent or coordination. Polymer macromolecules contain tens, hundreds, thousands or tens of thousands of atoms or repeating elementary units. Information about the polymer structure was obtained by studying the properties of solutions, the structure of crystals, and the mechanical and physicochemical properties of inorganic substances. In support of the above, it should be noted that there is a sufficient amount of scientific literature confirming the fact of the polymeric structure of some inorganic substances.
A logical remark would be: why is there so much information about synthetic organic polymers and so little about inorganic ones? If there are inorganic polymeric substances, what exactly are they and where are they used? Several examples of inorganic polymers were given above. These are well-known substances that everyone knows, but few people know that these substances can be classified as polymers. By and large, the average person doesn’t care whether graphite can be classified as a polymer or not; as for precious stones, for some it may even be offensive to equate expensive jewelry with cheap plastic jewelry. Nevertheless, if there is reason to call some inorganic substances polymers, then why not talk about it. Let's look at some representatives of such materials and look in more detail at the most interesting ones.
The synthesis of inorganic polymers most often requires very pure starting materials, as well as high temperatures and pressures. The main methods for their production, like organic polymers, are polymerization, polycondensation and polycoordination. The simplest inorganic polymers include homochain compounds consisting of chains or frameworks built from identical atoms. In addition to the well-known carbon, which is the main element involved in the construction of almost all organic polymers, other elements can also participate in the construction of macromolecules. These elements include boron from the third group, silicon, germanium and tin from the fourth group, which also includes carbon, phosphorus, arsenic, antimony and bismuth from the fifth group, sulfur, selenium, tellurium from the sixth. Mainly homochain polymers obtained from these elements are used in electronics and optics. The electronics industry is developing at a very high pace and the demand for synthetic crystals has long exceeded supply. Of particular note, however, is carbon and the inorganic polymers that are produced on its basis: diamond and graphite. Graphite is a well-known material that has found application in various industries. Pencils, electrodes, crucibles, paints, and lubricants are made from graphite. Thousands of tons of graphite go to the needs of the nuclear industry due to its properties to slow down neutrons. In the article we will dwell in more detail on the most interesting representatives of inorganic polymers - precious stones.
The most interesting, pretentious, women-loved representative of inorganic polymers are diamonds. Diamonds are very expensive minerals, which can also be classified as inorganic polymers; they are mined in nature by five large companies: DeBeers, Alrosa, Leviev, BHPBilliton, RioTinto. It was the DeBeers company that created the reputation of these stones. Smart marketing boils down to the slogan, “forever.” DeBeers has turned this stone into a symbol of love, prosperity, power, and success. An interesting fact is that diamonds are found quite often in nature, for example sapphires and rubies, which are rarer minerals, but they are valued lower than diamonds. The most interesting thing is the situation that has developed in the natural diamond market. The fact is that there are technologies that make it possible to obtain synthetic diamonds. In 1954, General Electric researcher Tracy Hall invented a device that made it possible to obtain diamond crystals from iron sulfide at a pressure of 100,000 atmospheres and a temperature of over 2500ºC. The quality of these stones was not high from a jewelry point of view, but the hardness was the same as that of natural stone. Hall's invention was improved and in 1960 General Electric created a plant in which it was possible to produce gem-quality diamonds. The negative point was that the price of synthetic stones was higher than natural ones.
At the moment, there are two technologies for synthesizing diamonds. HPHT (high pressure/high temperature) technology is the synthesis of diamonds in a combination of high pressure and high temperature. CVD (chemical vapor deposition) technology is a chemical vapor deposition technology that is considered more progressive and allows you to grow diamond, as if simulating the natural conditions of its growth. Both technologies have advantages and disadvantages. Campaigns using them solve the shortcomings of technology by using their own inventions and developments. For example, back in 1989, a group of Soviet scientists from Novosibirsk managed to reduce the fusion pressure to 60,000 atmospheres. After the collapse of the Soviet Union, developments in the field of diamond synthesis continued, thanks to many foreign investors interested in obtaining the technology for cheap synthesis of high-quality gemstones. For example, DeBeers, in order not to lose the opportunity to control the market, financed the work of some scientists. Some private entrepreneurs bought diamond synthesis equipment in Russia, for example, the now thriving American company Gemesis began by purchasing a diamond growing installation in Russia in 1996 for $60,000. Now Gemesis produces and sells diamonds of rare colors: yellow and blue, and the difference in price between these and exactly the same natural stones reaches 75%.
Another large company synthesizing diamonds, Apollo Diamond, is improving HPHT technology by synthesizing stones in a gas atmosphere of a certain composition (symbiosis technology of HPHT and CVD). This method brings Apollo Diamond to the market of jewelry stones; at the same time, the quality of synthetic diamonds grown using this technology is very high. It is increasingly difficult for gemotologists to distinguish synthetic stones from natural ones. This requires a complex of analyzes using fairly complex and expensive equipment. Apollo Diamond synthetic gem diamonds are almost impossible to distinguish from natural minerals using standard analysis methods.
World diamond production is now 115 million carats or 23 tons per year. Theoretically, this gigantic market could collapse and the reputation of diamonds as precious stones would be lost forever. Monopoly firms invest in stabilizing the situation and controlling the market. For example, expensive marketing campaigns are carried out, patents for artificial diamond manufacturing technologies are purchased so that these technologies are never introduced, certificates and quality passports are issued for branded diamonds confirming their natural origin. But will this hold back the progress of fusion technology?
Having talked about diamonds, we were distracted by the brilliance of the precious stones of the jewelry industry, but we should also point out industrial stones. In this case, most diamond-growing enterprises operate primarily for the needs of the electronics and optical industries. The industrial stone market may not be as intriguing as the jewelry market, but it is nonetheless huge. For example, Apollo Diamond's main income is the synthesis of thin diamond disks for semiconductors. By the way, now a diamond synthesis installation with a productivity of about 200 kg of diamonds per month can be purchased for 30 thousand dollars.
Another representative of precious stones is ruby. The first synthetic ruby was born in 1902. It was synthesized by the French engineer Verneuil by melting aluminum oxide and chromium powder, which then crystallized into a six-gram ruby. This simplicity of synthesis allowed the relatively rapid development of industrial production of rubies around the world. This stone is in great demand. Every year, about 5 tons of rubies are mined in the world, and market needs amount to hundreds of tons. Rubies are needed in the watch industry and in the production of lasers. The technology proposed by Verneuil subsequently provided the prerequisites for the synthesis of sapphires and garnets. The largest productions of artificial rubies are located in France, Switzerland, Germany, Great Britain, and the USA. The economics of production are as follows. The lion's share of the cost is consumed by energy costs. At the same time, the cost of synthesizing a kilogram of rubies is $60, the cost of a kilogram of sapphires is $200. The profitability of such a business is very high, since the purchase price for crystals is at least twice as high. Here a number of factors should be taken into account, such as the fact that the larger the grown single crystal, the lower its cost; also, when producing products from crystals, their price will be much higher than the price of sold crystals (for example, the production and sale of glass). As for equipment, Russian installations for growing crystals cost about 50 thousand dollars, Western ones are an order of magnitude more expensive, while the payback period for organized production is on average two years. As already mentioned, the market needs for synthetic crystals are colossal. For example, sapphire crystals are in great demand. About a thousand tons of sapphires are synthesized around the world per year. Annual production needs reach a million tons!
Emeralds are synthesized exclusively for the needs of the jewelry industry. Unlike other crystals, emerald is obtained not from a melt, but from a solution of boron ahydride at a temperature of 400 ° C and a pressure of 500 atmospheres in a hydrothermal chamber. It is curious that the extraction of natural stone is only 500 kilograms per year. Synthetic emeralds are also produced in the world in quantities that are not as large as other crystals, about a ton per year. The fact is that the technology for synthesizing emeralds is low-productivity, but the profitability of such production is high. Producing about 5 kilograms of crystals per month at a cost of $200 per kilogram, the selling price of synthetic emeralds is almost equal to the price of natural ones. The cost of the installation for the synthesis of emeralds is about 10 thousand dollars.
But the most popular synthetic crystal is silicon. Perhaps it will give odds to any precious stone. At the moment, silicon occupies 80% of the total market for synthetic crystals. The market is experiencing a shortage of silicon due to the rapid development of high technologies. At the moment, the profitability of silicon production exceeds 100%. The price of a kilogram of silicon is about $100 per kilogram, while the cost of synthesis reaches $25.
Ultrapure silicon is used as a semiconductor. Solar photocells with a high efficiency are made from its crystals. Silicon, like carbon, can create long molecular chains from its atoms. In this way, silane and rubber are obtained, which have amazing properties. Several years ago, the whole world was excited by the news of the experiments of the American engineer Walter Robbs, who managed to produce a film of silicone rubber 0.0025 centimeters thick. He covered the cage in which the hamster lived with this rubber and lowered the hamster into the aquarium. For several hours, the world's first submarine hamster breathed oxygen dissolved in water, and was alert and showed no signs of anxiety. It turns out that the film plays the role of a membrane, performing the same functions as the gills of fish. The film allows life gas molecules inside, while carbon dioxide is forced out through the film. This discovery makes it possible to organize human life under water by moving aside cylinders with a breathing mixture and oxygen generators.
Silicon comes in three types: metallurgical silicon (MG), electronics grade silicon (EG), and solar grade silicon (SG). Due to a series of energy crises, alternative energy technologies are being intensively introduced. These include the conversion of solar energy into electrical energy, that is, the use of solar installations powered by solar batteries. An important component of solar cells is silicon. In Ukraine, the Zaporozhye titanium-magnesium plant produced silicon for solar panels. Under the Soviet Union, this enterprise produced 200 tons of silicon, with the all-Union production volume being 300 tons. The author currently knows nothing about the situation with silicon production in Zaporozhye. The cost of organizing a modern production of polycrystalline silicon for the needs of the energy industry with a capacity of 1000 tons per year is about 56 million dollars. The synthesis of silicon for various needs throughout the world ranks first in demand and will hold this position for a long time.
In the article we examined only some representatives of inorganic polymers. Perhaps many of the things told above were perceived by some with surprise and genuine interest. Someone took a fresh look at the concept of the philosopher's stone; even if it is not gold, it is still possible to obtain precious stones from nondescript metal oxides and other unremarkable substances. We hope that the article gave rise to thought and at least entertained the reader with interesting facts.
In nature, there are organoelement, organic and inorganic polymers. Inorganic materials include materials whose main chain is inorganic and whose side branches are not hydrocarbon radicals. Elements of groups III-VI of the periodic system of chemical elements are most prone to the formation of polymers of inorganic origin.
Classification
Organic and inorganic polymers are being actively studied, their new characteristics are being determined, so a clear classification of these materials has not yet been developed. However, certain groups of polymers can be distinguished.
Depending on the structure:
- linear;
- flat;
- branched;
- polymer meshes;
- three-dimensional and others.
Depending on the main chain atoms forming the polymer:
- homochain type (-M-)n - consist of one type of atom;
- heterochain type (-M-L-)n - consist of different types of atoms.
Depending on origin:
- natural;
- artificial.
To classify substances that are macromolecules in the solid state as inorganic polymers, it is also necessary to have a certain anisotropy in their spatial structure and corresponding properties.

Main Features
More common are heterochain polymers, in which there is an alternation of electropositive and electronegative atoms, for example B and N, P and N, Si and O. Heterochain inorganic polymers (HP) can be obtained using polycondensation reactions. The polycondensation of oxoanions is accelerated in an acidic environment, and the polycondensation of hydrated cations is accelerated in an alkaline environment. Polycondensation can be carried out either in solution or at high temperature.
Many of the heterochain inorganic polymers can only be obtained under high-temperature synthesis conditions, for example, directly from simple substances. The formation of carbides, which are polymer bodies, occurs when certain oxides interact with carbon, as well as in the presence of high temperature.
Long homochain chains (with a degree of polymerization n>100) form carbon and p-elements of group VI: sulfur, selenium, tellurium.

Inorganic polymers: examples and applications
The specificity of NP is the formation of polymer macromolecules with a regular three-dimensional structure. The presence of a rigid framework of chemical bonds provides such compounds with significant hardness.
This property allows the use of inorganic polymers. The use of these materials has found wide application in industry.
The exceptional chemical and thermal resistance of NP is also a valuable property. For example, reinforcing fibers made from organic polymers are stable in air up to a temperature of 150-220 °C. Meanwhile, boron fiber and its derivatives remain stable up to a temperature of 650˚C. This is why inorganic polymers are promising for creating new chemically and heat-resistant materials.
NPs, which at the same time have properties similar to organic ones and retain their specific properties, are also of practical importance. These include phosphates, polyphosphazenes, silicates, polymers with various side groups.

Carbon polymers
The task: “Give examples of inorganic polymers” is often found in chemistry textbooks. It is advisable to carry it out with mention of the most prominent NPs - carbon derivatives. After all, this includes materials with unique characteristics: diamonds, graphite and carbine.
Carbyne is an artificially created, little-studied linear polymer with unsurpassed strength indicators, not inferior to, and according to a number of studies, superior to graphene. However, carbyne is a mysterious substance. After all, not all scientists recognize its existence as an independent material.
Externally it looks like a metal-crystalline black powder. Has semiconductor properties. The electrical conductivity of carbyne increases significantly when exposed to light. It does not lose these properties even at temperatures up to 5000 °C, which is much higher than for other materials of similar purpose. The material was obtained in the 60s by V.V. Korshak, A.M. Sladkov, V.I. Kasatochkin and Yu.P. Kudryavtsev by catalytic oxidation of acetylene. The most difficult thing was to determine the type of bonds between carbon atoms. Subsequently, a substance with only double bonds between carbon atoms was obtained at the Institute of Organoelement Compounds of the USSR Academy of Sciences. The new compound was named polycumulene.
Graphite - in this ordering extends only in the plane. Its layers are connected not by chemical bonds, but by weak intermolecular interactions, so it conducts heat and current and does not transmit light. Graphite and its derivatives are fairly common inorganic polymers. Examples of their use: from pencils to the nuclear industry. By oxidizing graphite, intermediate oxidation products can be obtained.
Diamond - its properties are fundamentally different. Diamond is a spatial (three-dimensional) polymer. All carbon atoms are held together by strong covalent bonds. Therefore, this polymer is extremely durable. Diamond does not conduct current or heat and has a transparent structure.

Boron polymers
If you are asked what inorganic polymers you know, feel free to answer - boron polymers (-BR-). This is a fairly broad class of NPs, widely used in industry and science.
Boron carbide - its formula more correctly looks like this (B12C3)n. Its unit cell is rhombohedral. The framework is formed by twelve covalently bonded boron atoms. And in the middle of it is a linear group of three covalently bonded carbon atoms. The result is a very durable structure.
Borides - their crystals are formed similar to the carbide described above. The most stable of them is HfB2, which melts only at a temperature of 3250 °C. TaB2 has the greatest chemical resistance - it is not affected by either acids or their mixtures.
Boron nitride - it is often called white talc due to its similarity. This similarity is really only superficial. Structurally it is similar to graphite. It is obtained by heating boron or its oxide in an ammonia atmosphere.

Borazon
Elbor, borazon, cyborite, kingsongite, cubonite are superhard inorganic polymers. Examples of their application: production of abrasive materials, metal processing. These are chemically inert substances based on boron. Hardness is closer to that of other materials than diamonds. In particular, borazone leaves scratches on diamond, which also leaves scratches on borazone crystals.
However, these NPs have several advantages over natural diamonds: they have greater heat resistance (withstand temperatures up to 2000 °C, while diamond is destroyed at temperatures in the range of 700-800 °C) and high resistance to mechanical loads (they are not so fragile). Borazon was obtained at a temperature of 1350 °C and a pressure of 62,000 atmospheres by Robert Wentorf in 1957. Similar materials were obtained by Leningrad scientists in 1963.
Inorganic sulfur polymers
Homopolymer - this modification of sulfur has a linear molecule. The substance is not stable; when temperature fluctuates, it breaks up into octahedral cycles. Formed in the event of sudden cooling of molten sulfur.
Polymer modification of sulfur dioxide. Very similar to asbestos, has a fibrous structure.
Selenium polymers
Gray selenium is a polymer with helical linear macromolecules nested in parallel. In the chains, selenium atoms are linked covalently, and macromolecules are linked by molecular bonds. Even molten or dissolved selenium does not break down into individual atoms.
Red or amorphous selenium is also a polymer with a chain structure, but with a poorly ordered structure. In the temperature range of 70-90 ˚С it acquires rubber-like properties, turning into a highly elastic state, which resembles organic polymers.
Selenium carbide, or rock crystal. Thermally and chemically stable, fairly strong spatial crystal. Piezoelectric and semiconductor. It was obtained under artificial conditions by reacting coal in an electric furnace at a temperature of about 2000 °C.
Other selenium polymers:
- Monoclinic selenium is more ordered than amorphous red, but inferior to gray.
- Selenium dioxide, or (SiO2)n, is a three-dimensional network polymer.
- Asbestos is a polymer of selenium oxide with a fibrous structure.

Phosphorus polymers
There are many modifications of phosphorus: white, red, black, brown, purple. Red - NP of fine-crystalline structure. It is obtained by heating white phosphorus without air access at a temperature of 2500 ˚C. Black phosphorus was obtained by P. Bridgman under the following conditions: pressure 200,000 atmospheres at a temperature of 200 °C.
Phosphornitride chlorides are compounds of phosphorus with nitrogen and chlorine. The properties of these substances change with increasing mass. Namely, their solubility in organic substances decreases. When the molecular weight of the polymer reaches several thousand units, a rubber-like substance is formed. It is the only sufficiently heat-resistant carbon-free rubber. It is destroyed only at temperatures above 350 °C.
Conclusion
Inorganic polymers for the most part are substances with unique characteristics. They are used in production, in construction, for the development of innovative and even revolutionary materials. As the properties of known NPs are studied and new ones are created, the scope of their application is expanding.
Inorganic polymers
Inorganic polymers- polymers that do not contain C-C bonds in the repeating unit, but are capable of containing an organic radical as side substituents.

Classification of polymers
1. Homochain polymers Carbon and chalcogens (plastic modification of sulfur).

Mineral fiber asbestos

Characteristics of asbestos
Asbestos(Greek ἄσβεστος, - indestructible) is the collective name for a group of fine-fiber minerals from the class of silicates. Consist of the finest flexible fibers.
Ca2Mg5Si8O22(OH)2 - formula
The two main types of asbestos are serpentine asbestos (chrysotile asbestos, or white asbestos) and amphibole asbestos.

Chemical composition
In terms of their chemical composition, asbestos is aqueous silicates of magnesium, iron, and partly calcium and sodium. The following substances belong to the class of chrysotile asbestos:
Mg6(OH)8
2Na2O*6(Fe,Mg)O*2Fe2O3*17SiO2*3H2O

Safety
Asbestos is practically inert and does not dissolve in body fluids, but has a noticeable carcinogenic effect. People involved in asbestos mining and processing are several times more likely to develop tumors than the general population. Most often it causes lung cancer, tumors of the peritoneum, stomach and uterus.
Based on the results of comprehensive scientific research into carcinogens, the International Agency for Research on Cancer has classified asbestos as one of the most dangerous carcinogens in the first category.

Application of asbestos
Production of fire-resistant fabrics (including for sewing suits for firefighters).
In construction (as part of asbestos-cement mixtures for the production of pipes and slate).
In places where it is necessary to reduce the influence of acids.

The role of inorganic polymers in the formation of the lithosphere

Lithosphere
Lithosphere- the hard shell of the Earth. It consists of the earth's crust and the upper part of the mantle, up to the asthenosphere.
The lithosphere beneath the oceans and continents varies significantly. The lithosphere beneath the continents consists of sedimentary, granite and basalt layers with a total thickness of up to 80 km. The lithosphere under the oceans has undergone many stages of partial melting as a result of the formation of the oceanic crust, it is greatly depleted in fusible rare elements, mainly consists of dunites and harzburgites, its thickness is 5-10 km, and the granite layer is completely absent.


Chemical composition
The main components of the Earth's crust and surface soil of the Moon are Si and Al oxides and their derivatives. This conclusion can be made based on existing ideas about the prevalence of basalt rocks. The primary substance of the earth's crust is magma - a fluid form of rock that contains, along with molten minerals, a significant amount of gases. When magma reaches the surface, it forms lava, which solidifies into basalt rocks. The main chemical component of lava is silica, or silicon dioxide, SiO2. However, at high temperatures, silicon atoms can easily be replaced by other atoms, such as aluminum, forming various types of aluminosilicates. In general, the lithosphere is a silicate matrix with the inclusion of other substances formed as a result of physical and chemical processes that occurred in the past under conditions of high temperature and pressure. Both the silicate matrix itself and the inclusions in it contain predominantly substances in polymer form, that is, heterochain inorganic polymers.

Granite
Granite - silicic igneous intrusive rock. It consists of quartz, plagioclase, potassium feldspar and micas - biotite and muscovite. Granites are very widespread in the continental crust.
The largest volumes of granites are formed in collision zones, where two continental plates collide and thickening of the continental crust occurs. According to some researchers, a whole layer of granite melt is formed in the thickened collision crust at the level of the middle crust (depth 10-20 km). In addition, granitic magmatism is characteristic of active continental margins, and to a lesser extent, of island arcs.
Mineral composition of granite:
feldspars - 60-65%;
quartz - 25-30%;
dark-colored minerals (biotite, rarely hornblende) - 5-10%.

Basalt
Mineral composition. The groundmass is composed of microlites of plagioclase, clinopyroxene, magnetite or titanomagnetite, as well as volcanic glass. The most common accessory mineral is apatite.
Chemical composition. The silica content (SiO2) ranges from 45 to 52-53%, the sum of alkaline oxides Na2O+K2O up to 5%, in alkaline basalts up to 7%. Other oxides can be distributed as follows: TiO2 = 1.8-2.3%; Al2O3=14.5-17.9%; Fe2O3=2.8-5.1%; FeO=7.3-8.1%; MnO=0.1-0.2%; MgO=7.1-9.3%; CaO=9.1-10.1%; P2O5=0.2-0.5%;

Quartz (Silicon(IV) Oxide, Silica)

Formula: SiO2
Formula: SiO2
Color: colorless, white, violet, gray, yellow, brown
Trait color: white
Shine: glassy, sometimes greasy in solid masses
Density: 2.6-2.65 g/cm³
Hardness: 7




Chemical properties




Corundum (Al2O3, alumina)

Formula: Al2O3
Formula: Al2O3
Color: blue, red, yellow, brown, gray
Trait color: white
Shine: glass
Density: 3.9-4.1 g/cm³
Hardness: 9






Tellurium

Tellurium chain structure
Crystals are hexagonal, the atoms in them form helical chains and are connected by covalent bonds to their nearest neighbors. Therefore, elemental tellurium can be considered an inorganic polymer. Crystalline tellurium is characterized by a metallic luster, although due to its complex of chemical properties it can rather be classified as a non-metal.

Applications of tellurium
Production of semiconductor materials
Rubber production
High temperature superconductivity

Selenium

Selenium chain structure
Black Gray Red

Gray selenium
Gray selenium (sometimes called metallic) has crystals in a hexagonal system. Its elementary lattice can be represented as a slightly deformed cube. All its atoms seem to be strung on spiral chains, and the distances between neighboring atoms in one chain are approximately one and a half times less than the distance between the chains. Therefore, the elementary cubes are distorted.

Applications of gray selenium
Ordinary gray selenium has semiconducting properties; it is a p-type semiconductor, i.e. conductivity in it is created mainly not by electrons, but by “holes”.
Another practically very important property of semiconductor selenium is its ability to sharply increase electrical conductivity under the influence of light. The action of selenium photocells and many other devices is based on this property.

Red selenium
Red selenium is a less stable amorphous modification.
A polymer with a chain structure but a poorly ordered structure. In the temperature range of 70-90°C, it acquires rubber-like properties, turning into a highly elastic state.
Does not have a specific melting point.
Red amorphous selenium with increasing temperature (-55) it begins to transform into gray hexagonal selenium

Sulfur


Structural features
The plastic modification of sulfur is formed by helical chains of sulfur atoms with left and right axes of rotation. These chains are twisted and pulled in one direction.
Plastic sulfur is unstable and spontaneously turns into rhombic sulfur.


Obtaining plastic sulfur

Application of sulfur
Preparation of sulfuric acid;
In the paper industry;
in agriculture (to combat plant diseases, mainly grapes and cotton);
in the production of dyes and luminous compositions;
to obtain black (hunting) powder;
in the production of matches;
ointments and powders for the treatment of certain skin diseases.

Allotropic modifications of carbon

Comparative characteristics

Application of allotropic modifications of carbon
Diamond - in industry: it is used to make knives, drills, cutters; in jewelry making. The future is the development of microelectronics on diamond substrates.
Graphite – for the manufacture of melting crucibles, electrodes; plastic filler; neutron moderator in nuclear reactors; component of the composition for the manufacture of leads for black graphite pencils (mixed with kaolin)

Mineral fiber asbestos

Characteristics of asbestos
Asbestos(Greek ἄσβεστος, - indestructible) is the collective name for a group of fine-fiber minerals from the class of silicates. Consist of the finest flexible fibers.
Ca2Mg5Si8O22(OH)2 - formula
The two main types of asbestos are serpentine asbestos (chrysotile asbestos, or white asbestos) and amphibole asbestos.

Chemical composition
In terms of their chemical composition, asbestos is aqueous silicates of magnesium, iron, and partly calcium and sodium. The following substances belong to the class of chrysotile asbestos:
Mg6(OH)8
2Na2O*6(Fe,Mg)O*2Fe2O3*17SiO2*3H2O

Safety
Asbestos is practically inert and does not dissolve in body fluids, but has a noticeable carcinogenic effect. People involved in asbestos mining and processing are several times more likely to develop tumors than the general population. Most often it causes lung cancer, tumors of the peritoneum, stomach and uterus.
Based on the results of comprehensive scientific research into carcinogens, the International Agency for Research on Cancer has classified asbestos as one of the most dangerous carcinogens in the first category.

Application of asbestos
Production of fire-resistant fabrics (including for sewing suits for firefighters).
In construction (as part of asbestos-cement mixtures for the production of pipes and slate).
In places where it is necessary to reduce the influence of acids.

The role of inorganic polymers in the formation of the lithosphere

Lithosphere
Lithosphere- the hard shell of the Earth. It consists of the earth's crust and the upper part of the mantle, up to the asthenosphere.
The lithosphere beneath the oceans and continents varies significantly. The lithosphere beneath the continents consists of sedimentary, granite and basalt layers with a total thickness of up to 80 km. The lithosphere under the oceans has undergone many stages of partial melting as a result of the formation of the oceanic crust, it is greatly depleted in fusible rare elements, mainly consists of dunites and harzburgites, its thickness is 5-10 km, and the granite layer is completely absent.


Chemical composition
The main components of the Earth's crust and surface soil of the Moon are Si and Al oxides and their derivatives. This conclusion can be made based on existing ideas about the prevalence of basalt rocks. The primary substance of the earth's crust is magma - a fluid form of rock that contains, along with molten minerals, a significant amount of gases. When magma reaches the surface, it forms lava, which solidifies into basalt rocks. The main chemical component of lava is silica, or silicon dioxide, SiO2. However, at high temperatures, silicon atoms can easily be replaced by other atoms, such as aluminum, forming various types of aluminosilicates. In general, the lithosphere is a silicate matrix with the inclusion of other substances formed as a result of physical and chemical processes that occurred in the past under conditions of high temperature and pressure. Both the silicate matrix itself and the inclusions in it contain predominantly substances in polymer form, that is, heterochain inorganic polymers.

Granite
Granite - silicic igneous intrusive rock. It consists of quartz, plagioclase, potassium feldspar and micas - biotite and muscovite. Granites are very widespread in the continental crust.
Mineral composition of granite:
feldspars - 60-65%;
quartz - 25-30%;
dark-colored minerals (biotite, rarely hornblende) - 5-10%.
The largest volumes of granites are formed in collision zones, where two continental plates collide and thickening of the continental crust occurs. According to some researchers, a whole layer of granite melt is formed in the thickened collision crust at the level of the middle crust (depth 10-20 km). In addition, granitic magmatism is characteristic of active continental margins, and to a lesser extent, of island arcs.

Basalt
Mineral composition. The groundmass is composed of microlites of plagioclase, clinopyroxene, magnetite or titanomagnetite, as well as volcanic glass. The most common accessory mineral is apatite.
Chemical composition. The silica content (SiO2) ranges from 45 to 52-53%, the sum of alkaline oxides Na2O+K2O up to 5%, in alkaline basalts up to 7%. Other oxides can be distributed as follows: TiO2 = 1.8-2.3%; Al2O3=14.5-17.9%; Fe2O3=2.8-5.1%; FeO=7.3-8.1%; MnO=0.1-0.2%; MgO=7.1-9.3%; CaO=9.1-10.1%; P2O5=0.2-0.5%;

Quartz (Silicon(IV) Oxide, Silica)

Formula: SiO2
Formula: SiO2
Color: colorless, white, violet, gray, yellow, brown
Trait color: white
Shine: glassy, sometimes greasy in solid masses
Density: 2.6-2.65 g/cm³
Hardness: 7




Chemical properties




Corundum (Al2O3, alumina)

Formula: Al2O3
Formula: Al2O3
Color: blue, red, yellow, brown, gray
Trait color: white
Shine: glass
Density: 3.9-4.1 g/cm³
Hardness: 9






Tellurium

Tellurium chain structure
Crystals are hexagonal, the atoms in them form helical chains and are connected by covalent bonds to their nearest neighbors. Therefore, elemental tellurium can be considered an inorganic polymer. Crystalline tellurium is characterized by a metallic luster, although due to its complex of chemical properties it can rather be classified as a non-metal.

Applications of tellurium
Production of semiconductor materials
Rubber production
High temperature superconductivity

Selenium

Selenium chain structure
Black Gray Red

Gray selenium
Gray selenium (sometimes called metallic) has crystals in a hexagonal system. Its elementary lattice can be represented as a slightly deformed cube. All its atoms seem to be strung on spiral chains, and the distances between neighboring atoms in one chain are approximately one and a half times less than the distance between the chains. Therefore, the elementary cubes are distorted.

Applications of gray selenium
Ordinary gray selenium has semiconducting properties; it is a p-type semiconductor, i.e. conductivity in it is created mainly not by electrons, but by “holes”.
Another practically very important property of semiconductor selenium is its ability to sharply increase electrical conductivity under the influence of light. The action of selenium photocells and many other devices is based on this property.

Red selenium
Red selenium is a less stable amorphous modification.
A polymer with a chain structure but a poorly ordered structure. In the temperature range of 70-90°C, it acquires rubber-like properties, turning into a highly elastic state.
Does not have a specific melting point.
Red amorphous selenium with increasing temperature (-55) it begins to transform into gray hexagonal selenium

Sulfur


Structural features
The plastic modification of sulfur is formed by helical chains of sulfur atoms with left and right axes of rotation. These chains are twisted and pulled in one direction.
Plastic sulfur is unstable and spontaneously turns into rhombic sulfur.


Obtaining plastic sulfur

Application of sulfur
Preparation of sulfuric acid;
In the paper industry;
in agriculture (to combat plant diseases, mainly grapes and cotton);
in the production of dyes and luminous compositions;
to obtain black (hunting) powder;
in the production of matches;
ointments and powders for the treatment of certain skin diseases.

Allotropic modifications of carbon

Comparative characteristics

Application of allotropic modifications of carbon
Diamond - in industry: it is used to make knives, drills, cutters; in jewelry making. The future is the development of microelectronics on diamond substrates.
Graphite – for the manufacture of melting crucibles, electrodes; plastic filler; neutron moderator in nuclear reactors; component of the composition for the manufacture of leads for black graphite pencils (mixed with kaolin)
Gray selenium (sometimes called metallic) has crystals in a hexagonal system. Its elementary lattice can be represented as a slightly deformed cube. All its atoms seem to be strung on spiral chains, and the distances between neighboring atoms in one chain are approximately one and a half times less than the distance between the chains. Therefore, the elementary cubes are distorted.
Ordinary gray selenium has semiconducting properties; it is a p-type semiconductor, i.e. conductivity in it is created mainly not by electrons, but by “holes”.
Another practically very important property of semiconductor selenium is its ability to sharply increase electrical conductivity under the influence of light. The action of selenium photocells and many other devices is based on this property.
Red selenium is a less stable amorphous modification.
A polymer with a chain structure but a poorly ordered structure. In the temperature range of 70-90°C, it acquires rubber-like properties, turning into a highly elastic state.
Does not have a specific melting point.
Red amorphous selenium with increasing temperature (-55) it begins to transform into gray hexagonal selenium
The plastic modification of sulfur is formed by helical chains of sulfur atoms with left and right axes of rotation. These chains are twisted and pulled in one direction.
Plastic sulfur is unstable and spontaneously turns into rhombic sulfur.
Preparation of sulfuric acid;
In the paper industry;
in agriculture (to combat plant diseases, mainly grapes and cotton);
in the production of dyes and luminous compositions;
to obtain black (hunting) powder;
in the production of matches;
ointments and powders for the treatment of certain skin diseases.
Diamond - in industry: it is used to make knives, drills, cutters; in jewelry making. The future is the development of microelectronics on diamond substrates.
Graphite – for the manufacture of melting crucibles, electrodes; plastic filler; neutron moderator in nuclear reactors; component of the composition for the manufacture of leads for black graphite pencils (mixed with kaolin)
Polymers are high-molecular compounds consisting of many repeating atomic groups of different or identical structures - units. These links are interconnected by coordination or chemical bonds into branched or long linear chains and into spatial three-dimensional structures.
Polymers are:
- synthetic,
- artificial,
- organic.
Organic polymers are formed in nature in animal and plant organisms. The most important of them are proteins, polysaccharides, nucleic acids, rubber and other natural compounds.
Man has long and widely used organic polymers in his daily life. Leather, wool, cotton, silk, fur - all this is used to produce clothing. Lime, cement, clay, organic glass (plexiglass) - in construction.
Organic polymers are also present in humans. For example, nucleic acids (also called DNA), as well as ribonucleic acids (RNA).
Properties of organic polymers
All organic polymers have special mechanical properties:
- low fragility of crystalline and glassy polymers (organic glass, plastics);
- elasticity, that is, high reversible deformation under small loads (rubber);
- orientation of macromolecules under the action of a directed mechanical field (production of films and fibers);
- at low concentrations, the viscosity of solutions is high (polymers first swell and then dissolve);
- under the influence of a small amount of reagent they can quickly change their physical and mechanical characteristics (for example, leather tanning, rubber vulcanization).
Table 1. Combustion characteristics of some polymers.
| Polymers | Behavior of the material when introduced into the flame and flammability | Character of the flame | Smell |
|---|---|---|---|
| Polyethylene (PE) | It melts drop by drop, burns well, and continues to burn when removed from the flame. | Glowing, initially bluish, then yellow | Burning paraffin |
| Polypropylene (PP) | Same | Same | Same |
| Polycarbonate (PC) | Same | Smoking | |
| Polyamide (PA) | Burns, flows like a thread | Bluish below, with yellow edges | Singed hair or burnt plants |
| Polyurethane (PU) | Burns, flows drop by drop | Yellow, bluish below, glowing, gray smoke | Harsh, unpleasant |
| Polystyrene (PS) | Self-ignites, melts | Bright yellow, glowing, smoky | Sweetish floral, with a hint of styrene scent |
| Polyethylene terephthalate (PET) | Burning, dripping | Yellow-orange, smoky | Sweet, fragrant |
| Epoxy resin (ED) | Burns well, continues to burn when removed from flame | Yellow smoky | Specific fresh (at the very beginning of heating) |
| Polyester resin (PN) | Burns, charred | Glowing, smoky, yellow | Sweetish |
| Rigid polyvinyl chloride (PVC) | Burns with difficulty and scattering, when removed from the flame it goes out and softens | Bright green | Acute, hydrogen chloride |
| PVC plasticized | Burns with difficulty and when removed from the flame, with scattering | Bright green | Acute, hydrogen chloride |
| Phenol-formaldehyde resin (FFR) | Difficult to light, burns poorly, retains its shape | Yellow | Phenol, formaldehyde |
Table 2. Solubility of polymer materials.
Table 3. Coloring of polymers according to the Lieberman-Storch-Moravsky reaction.
Articles on the topic
Among most materials, the most popular and widely known are polymer composite materials (PCMs). They are actively used in almost every area of human activity. It is these materials that are the main component for the manufacture of various products used for completely different purposes, from fishing rods and boat hulls, to cylinders for storing and transporting flammable substances, as well as helicopter rotor blades. Such wide popularity of PCM is associated with the ability to solve technological problems of any complexity associated with the production of composites with certain properties, thanks to the development of polymer chemistry and methods for studying the structure and morphology of polymer matrices that are used in the production of PCM.