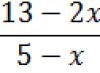We list the most important applications of semiconductors:
1) semiconductor diodes and triodes replace vacuum tubes with great success, as they are more economical, compact, simple in design, reliable, mechanically durable and have a long service life. Selenium rectifiers used in electrical and radio engineering have an efficiency of up to 70%, germanium rectifiers - up to 98%. There are rectifiers that operate at high temperatures. Semiconductor triodes have efficiency up to 50% (while vacuum tubes have efficiency of about 1%). Semiconductor devices consume little energy and require low voltage (compared to vacuum tubes), so the power supplies they require can be very small. This made it possible to solve a number of important problems in radio engineering (creation of miniature radio receivers and transmitters, etc.);
2) photoresistance - semiconductors (selenium, cadmium and lead sulfide, etc.), in which the electrical resistance sharply decreases when irradiated with light, ultraviolet, x-rays and other rays; they are used to measure light fluxes, illumination, reproduce sound recorded on film in various control, alarm, automatic control devices, etc. There are photoresistors that are sensitive to infrared radiation;
3) thermistors - semiconductors (mixtures of oxides of various metals: magnesium, nickel, titanium, etc.), whose electrical resistance strongly depends on temperature; they are used to measure temperatures (under conditions in which other methods are not applicable: chemically active environment, the presence of vibrations, the need for very small sensor sizes, etc.), for automatic temperature control, as limiters of the initial current value in starting devices and etc.;
4) varistors - semiconductors (silicon carbide, etc.), in which the electrical resistance strongly depends on the strength of the applied electric field; are used to protect electrical circuits from irregular high overvoltages, for example from lightning discharges.
In electric furnaces, instead of expensive and short-lived metal spirals, rods made of refractory semiconductors are used, allowing heating up to 1300 ° C. Thermoelements, composed of two semiconductors with and -conductivity, have
higher value of thermoelectromotive force coefficient. They can be used as converters of heat directly into electrical energy (with efficiency reaching up to 10%), as well as when using the Peltier effect, for cooling purposes (semiconductor thermoelectric generators and refrigerators).
The most famous semiconductor is silicon (Si). But besides him, there are many others. An example is such natural semiconductor materials as zinc blende (ZnS), cuprite (Cu 2 O), galena (PbS) and many others. The family of semiconductors, including semiconductors synthesized in laboratories, represent one of the most versatile classes of materials known to man.
Characteristics of semiconductors
Of the 104 elements of the periodic table, 79 are metals, 25 are non-metals, of which 13 have semiconductor properties and 12 have dielectric properties. The main difference between semiconductors is that their electrical conductivity increases significantly with increasing temperature. At low temperatures they behave like dielectrics, and at high temperatures they behave like conductors. This is how semiconductors differ from metals: the resistance of a metal increases in proportion to the increase in temperature.
Another difference between a semiconductor and a metal is that the resistance of a semiconductor drops under the influence of light, while the latter does not affect a metal. The conductivity of semiconductors also changes when a small amount of impurity is introduced.
Semiconductors are found among chemical compounds with a variety of crystal structures. These can be elements such as silicon and selenium, or binary compounds such as gallium arsenide. Many polyacetylene (CH) n, - semiconductor materials. Some semiconductors exhibit magnetic (Cd 1-x Mn x Te) or ferroelectric properties (SbSI). Others, with sufficient doping, become superconductors (GeTe and SrTiO 3). Many of the recently discovered high-temperature superconductors have non-metallic semiconducting phases. For example, La 2 CuO 4 is a semiconductor, but upon formation of an alloy with Sr it becomes a superconductor (La 1-x Sr x) 2 CuO 4.
Physics textbooks define a semiconductor as a material with an electrical resistance from 10 -4 to 10 7 Ohm m. An alternative definition is also possible. The band gap of the semiconductor is from 0 to 3 eV. Metals and semimetals are materials with zero energy gap, and substances in which it exceeds 3 eV are called insulators. There are exceptions. For example, semiconductor diamond has a bandgap width of 6 eV, semi-insulating GaAs - 1.5 eV. GaN, a material for the blue region, has a band gap of 3.5 eV.
Energy gap
The valence orbitals of atoms in a crystal lattice are divided into two groups of energy levels - the free band, located at the highest level and determining the electrical conductivity of semiconductors, and the valence band, located below. These levels, depending on the symmetry of the crystal lattice and the composition of the atoms, can intersect or be located at a distance from each other. In the latter case, an energy gap or, in other words, a forbidden zone appears between the zones.
The location and filling of the levels determines the electrical conductivity properties of the substance. Based on this criterion, substances are divided into conductors, insulators and semiconductors. The band gap of a semiconductor varies between 0.01-3 eV, and the energy gap of the dielectric exceeds 3 eV. Metals do not have energy gaps due to the overlap of levels.
Semiconductors and dielectrics, as opposed to metals, have a valence band filled with electrons, and the nearest free band, or conduction band, is fenced off from the valence band by an energy gap - a region of forbidden electron energies.
In dielectrics, thermal energy or a small electric field is not enough to make a jump through this gap; electrons do not enter the conduction band. They are not able to move along the crystal lattice and become carriers of electric current.
To initiate electrical conductivity, an electron at the valence level must be given energy that would be sufficient to overcome the energy gap. Only by absorbing an amount of energy no less than the size of the energy gap will the electron move from the valence level to the conduction level.
If the width of the energy gap exceeds 4 eV, excitation of the conductivity of the semiconductor by irradiation or heating is practically impossible - the excitation energy of electrons at the melting temperature is insufficient to jump through the energy gap zone. When heated, the crystal will melt until electronic conduction occurs. Such substances include quartz (dE = 5.2 eV), diamond (dE = 5.1 eV), and many salts.

Impurity and intrinsic conductivity of semiconductors
Pure semiconductor crystals have their own conductivity. Such semiconductors are called intrinsic semiconductors. An intrinsic semiconductor contains an equal number of holes and free electrons. When heated, the intrinsic conductivity of semiconductors increases. At a constant temperature, a state of dynamic equilibrium arises in the number of electron-hole pairs formed and the number of recombining electrons and holes, which remain constant under given conditions.
The presence of impurities has a significant effect on the electrical conductivity of semiconductors. Adding them allows you to greatly increase the number of free electrons with a small number of holes and increase the number of holes with a small number of electrons at the conduction level. Impurity semiconductors are conductors with impurity conductivity.
Impurities that easily give up electrons are called donor impurities. Donor impurities can be chemical elements with atoms whose valence levels contain more electrons than the atoms of the base substance. For example, phosphorus and bismuth are donor impurities of silicon.
The energy required for an electron to jump into the conduction region is called activation energy. Impurity semiconductors need much less of it than the main substance. With slight heating or illumination, mainly the electrons of the atoms of impurity semiconductors are released. A hole takes the place of the electron that leaves the atom. But the recombination of electrons into holes practically does not occur. The hole conductivity of the donor is negligible. This happens because the small number of impurity atoms prevents free electrons from frequently approaching the hole and occupying it. Electrons are located near holes, but are not able to fill them due to insufficient energy level.
A slight addition of a donor impurity increases the number of conduction electrons by several orders of magnitude compared to the number of free electrons in the native semiconductor. Electrons here are the main carriers of charges of atoms of impurity semiconductors. These substances are classified as n-type semiconductors.
Impurities that bind the electrons of a semiconductor, increasing the number of holes in it, are called acceptor impurities. Acceptor impurities are chemical elements with fewer electrons at the valence level than the base semiconductor. Boron, gallium, indium are acceptor impurities for silicon.
The characteristics of a semiconductor depend on the defects in its crystal structure. This is the reason for the need to grow extremely pure crystals. The conductivity parameters of the semiconductor are controlled by adding dopants. Silicon crystals are doped with phosphorus (a subgroup V element), which is a donor, to create an n-type silicon crystal. To obtain a crystal with hole conductivity, the acceptor boron is introduced into silicon. Semiconductors with a compensated Fermi level to move it to the middle of the band gap are created in a similar way.

Single element semiconductors
The most common semiconductor is, of course, silicon. Together with germanium, it became the prototype for a wide class of semiconductors that have similar crystal structures.
Si and Ge are the same as diamond and α-tin. In it, each atom is surrounded by 4 nearest atoms, which form a tetrahedron. This coordination is called quadruple coordination. Tetradrical bonded crystals have become fundamental to the electronics industry and play a key role in modern technology. Some elements of group V and VI of the periodic table are also semiconductors. Examples of this type of semiconductor are phosphorus (P), sulfur (S), selenium (Se) and tellurium (Te). In these semiconductors, atoms can have threefold (P), twofold (S, Se, Te) or fourfold coordination. As a result, similar elements can exist in several different crystal structures and can also be produced in the form of glass. For example, Se has been grown in monoclinic and trigonal crystal structures or as glass (which can also be considered a polymer).
Diamond has excellent thermal conductivity, excellent mechanical and optical characteristics, and high mechanical strength. The energy gap width is dE = 5.47 eV.
Silicon is a semiconductor used in solar cells, and in amorphous form in thin-film solar cells. It is the most used semiconductor in photovoltaic cells, is easy to manufacture, and has good electrical and mechanical properties. dE = 1.12 eV.
Germanium is a semiconductor used in gamma spectroscopy and high-efficiency photovoltaic cells. Used in the first diodes and transistors. Requires less cleaning than silicon. dE = 0.67 eV.
Selenium is a semiconductor that is used in selenium rectifiers, which have high radiation resistance and self-healing ability.

Two-element connections
The properties of semiconductors formed by elements of groups 3 and 4 of the periodic table resemble 4 groups. Transition from group 4 of elements to compounds of group 3-4. makes the bonds partially ionic due to the transfer of electron charge from the atom of group 3 to the atom of group 4. Ionicity changes the properties of semiconductors. It is the reason for the increase in the Coulomb interionic interaction and the energy of the energy break in the band structure of electrons. An example of a binary compound of this type is indium antimonide InSb, gallium arsenide GaAs, gallium antimonide GaSb, indium phosphide InP, aluminum antimonide AlSb, gallium phosphide GaP.
Ionicity increases, and its value grows even more in compounds of substances of groups 2-6, such as cadmium selenide, zinc sulfide, cadmium sulfide, cadmium telluride, zinc selenide. As a result, most compounds of groups 2–6 have a band gap wider than 1 eV, except for mercury compounds. Mercury telluride is a semiconductor without an energy gap, a semimetal, like α-tin.
Semiconductors of groups 2-6 with a large energy gap are used in the production of lasers and displays. Binary compounds of groups 2-6 with a narrowed energy gap are suitable for infrared receivers. Binary compounds of elements of groups 1-7 (copper bromide CuBr, silver iodide AgI, copper chloride CuCl) due to their high ionicity have a band gap wider than 3 eV. They are actually not semiconductors, but insulators. An increase in the cohesion energy of the crystal due to Coulomb interionic interaction promotes the structuring of atoms with sixfold rather than quadratic coordination. Compounds of groups 4-6 - lead sulfide and telluride, tin sulfide - are also semiconductors. The degree of ionicity of these substances also contributes to the formation of sixfold coordination. Significant ionicity does not prevent them from having very narrow band gaps, which allows them to be used for receiving IR radiation. Gallium nitride, a compound of 3-5 groups with a wide energy gap, has found application in LEDs operating in the blue part of the spectrum.
GaAs, gallium arsenide, is the second most popular semiconductor after silicon, commonly used as a substrate for other conductors, such as GaInNAs and InGaAs, in IR LEDs, high-frequency chips and transistors, high-efficiency photovoltaic cells, laser diodes, and nuclear radiation detectors. dE = 1.43 eV, which makes it possible to increase the power of devices compared to silicon. It is fragile, contains more impurities, and is difficult to manufacture.
ZnS, zinc sulfide, is a zinc salt of hydrogen sulfide with a band gap of 3.54 and 3.91 eV, used in lasers and as a phosphor.
SnS, tin sulfide - semiconductor used in photoresistors and photodiodes, dE= 1.3 and 10 eV.

Oxides
Metal oxides are generally excellent insulators, but there are exceptions. Examples of this type of semiconductors are nickel oxide, copper oxide, cobalt oxide, copper dioxide, iron oxide, europium oxide, zinc oxide. Since copper dioxide exists in the form of the mineral cuprite, its properties have been extensively studied. The procedure for growing this type of semiconductor is not yet fully understood, so their use is still limited. An exception is zinc oxide (ZnO), a compound of groups 2-6, used as a converter and in the production of adhesive tapes and adhesives.
The situation changed dramatically after superconductivity was discovered in many compounds of copper with oxygen. The first high-temperature superconductor discovered by Müller and Bednorz was a compound based on the semiconductor La 2 CuO 4 with an energy gap of 2 eV. By replacing trivalent lanthanum with divalent barium or strontium, hole charge carriers are introduced into the semiconductor. Achieving the required hole concentration turns La 2 CuO 4 into a superconductor. At this time, the highest temperature of transition to the superconducting state belongs to the compound HgBaCa 2 Cu 3 O 8. At high pressure its value is 134 K.
ZnO, zinc oxide, is used in varistors, blue LEDs, gas sensors, biological sensors, window coatings to reflect infrared light, as a conductor in LCD displays and solar cells. dE=3.37 eV.
Layered crystals
Binary compounds like lead diiodide, gallium selenide and molybdenum disulfide are distinguished by their layered crystal structure. Significant forces act in the layers, much stronger than the van der Waals bonds between the layers themselves. Semiconductors of this type are interesting because electrons behave quasi-two-dimensionally in the layers. The interaction of layers is changed by the introduction of third-party atoms - intercalation.
MoS 2, molybdenum disulfide is used in high-frequency detectors, rectifiers, memristors, transistors. dE=1.23 and 1.8 eV.

Organic semiconductors
Examples of semiconductors based on organic compounds are naphthalene, polyacetylene (CH 2) n, anthracene, polydiacetylene, phthalocyanides, polyvinylcarbazole. Organic semiconductors have an advantage over inorganic ones: they are easy to impart the desired qualities. Substances with conjugated bonds of the form -C=C-C= have significant optical nonlinearity and, due to this, are used in optoelectronics. In addition, the energy gap zones of organic semiconductors are changed by changing the compound formula, which is much easier than that of conventional semiconductors. Crystalline allotropes of carbon, fullerene, graphene, and nanotubes are also semiconductors.
Fullerene has a structure in the form of a convex closed polyhedron of an even number of carbon atoms. And doping fullerene C 60 with an alkali metal turns it into a superconductor.
Graphene is formed by a monatomic layer of carbon connected into a two-dimensional hexagonal lattice. Has record thermal conductivity and electron mobility, high rigidity
Nanotubes are graphite plates rolled into a tube, several nanometers in diameter. These forms of carbon have great promise in nanoelectronics. Depending on the adhesion, they may exhibit metallic or semiconductor qualities.

Magnetic semiconductors
Compounds with magnetic europium and manganese ions have interesting magnetic and semiconductor properties. Examples of this type of semiconductor are europium sulfide, europium selenide and solid solutions like Cd 1-x- Mn x Te. The content of magnetic ions affects how magnetic properties such as antiferromagnetism and ferromagnetism appear in substances. Semimagnetic semiconductors are solid magnetic solutions of semiconductors that contain magnetic ions in small concentrations. Such solid solutions attract attention due to their promise and great potential for possible applications. For example, unlike non-magnetic semiconductors, they can achieve a million times greater Faraday rotation.
The strong magneto-optical effects of magnetic semiconductors allow them to be used for optical modulation. Perovskites like Mn 0.7 Ca 0.3 O 3 have properties superior to the metal-semiconductor transition, the direct dependence of which on the magnetic field results in the phenomenon of giant magnetoresistance. They are used in radio engineering and optical devices that are controlled by a magnetic field, in waveguides of microwave devices.
Semiconductor ferroelectrics
This type of crystals is distinguished by the presence of electrical moments in them and the occurrence of spontaneous polarization. For example, such properties are possessed by the semiconductors lead titanate PbTiO 3 , barium titanate BaTiO 3 , germanium telluride GeTe, tin telluride SnTe, which at low temperatures have ferroelectric properties. These materials are used in nonlinear optical, storage devices and piezoelectric sensors.
Variety of semiconductor materials
Apart from the semiconductor substances mentioned above, there are many others that do not fall under any of the listed types. Compounds of elements with the formula 1-3-5 2 (AgGaS 2) and 2-4-5 2 (ZnSiP 2) form crystals in the structure of chalcopyrite. The bonds of the compounds are tetrahedral, similar to semiconductors of groups 3-5 and 2-6 with a zinc blende crystal structure. The compounds that form the elements of group 5 and 6 semiconductors (like As 2 Se 3) are semiconductor in the form of a crystal or glass. Bismuth and antimony chalcogenides are used in semiconductor thermoelectric generators. The properties of this type of semiconductor are extremely interesting, but they have not gained popularity due to their limited applications. However, the fact that they exist confirms the presence of still unexplored areas of semiconductor physics.
Semiconductors are characterized by both the properties of conductors and dielectrics. In semiconductor crystals, atoms establish covalent bonds (that is, one electron in a silicon crystal, like diamond, is connected by two atoms); electrons require a level of internal energy to be released from the atom (1.76 10 −19 J versus 11.2 10 −19 J, which characterizes the difference between semiconductors and dielectrics). This energy appears in them as the temperature increases (for example, at room temperature, the energy level of thermal motion of atoms is 0.4·10−19 J), and individual atoms receive energy to remove an electron from the atom. As the temperature increases, the number of free electrons and holes increases, therefore, in a semiconductor that does not contain impurities, the resistivity decreases. Conventionally, elements with an electron binding energy of less than 1.5-2 eV are considered semiconductors. The electron-hole conductivity mechanism manifests itself in native (that is, without impurities) semiconductors. It is called the intrinsic electrical conductivity of semiconductors.
Hole
When the bond between the electron and the nucleus is broken, a free space appears in the electron shell of the atom. This causes the transfer of an electron from another atom to an atom with a free place. The atom from which the electron passed receives another electron from another atom, etc. This is due to the covalent bonds of the atoms. Thus, a positive charge moves without moving the atom itself. This conditional positive charge is called a hole.
Self-density
At thermodynamic equilibrium, the electron density of a semiconductor is related to temperature by the following relationship:
- Planck's constant - electron mass - temperature; - level of the conductive band - Fermi level;Also, the hole density of a semiconductor is related to temperature as follows:
- Planck's constant; - hole mass; - temperature ; - Fermi level; - level of the valence band.The intrinsic density is related to and the following relationship:
Types of semiconductors
By the nature of conductivity
Self conductivity
Semiconductors in which free electrons and “holes” appear during the ionization of the atoms from which the entire crystal is built are called intrinsically conductive semiconductors. In semiconductors with intrinsic conductivity, the concentration of free electrons is equal to the concentration of “holes”.
Conductivity is related to particle mobility by the following relationship:
where is the resistivity, is the mobility of electrons, is the mobility of holes, is their concentration, q is the elementary electric charge (1.602·10 −19 C).
For an intrinsic semiconductor, the carrier concentrations coincide and the formula takes the form:
Impurity conductivity
To create semiconductor devices, crystals with impurity conductivity are often used. Such crystals are made by introducing impurities with atoms of a trivalent or pentavalent chemical element.
By type of conductivity
Electronic semiconductors (n-type)
n-type semiconductor
Term "n-type" comes from the word "negative", meaning the negative charge of the majority carriers. This type of semiconductor has an impurity nature. An impurity of a pentavalent semiconductor (for example, arsenic) is added to a tetravalent semiconductor (for example, silicon). During the interaction, each impurity atom enters into a covalent bond with silicon atoms. However, there is no place for the fifth electron of the arsenic atom in saturated valence bonds, and it goes to the outer electron shell. There, it takes less energy to remove an electron from an atom. The electron is stripped off and becomes free. In this case, charge transfer is carried out by an electron, not a hole, that is, this type of semiconductor conducts electric current like metals. Impurities that are added to semiconductors, causing them to become n-type semiconductors, are called donor impurities.
The conductivity of N-semiconductors is approximately equal to:
Hole semiconductors (p-type)
p-type semiconductor
Term "p-type" comes from the word “positive”, denoting the positive charge of the main carriers. This type of semiconductor, in addition to the impurity base, is characterized by the hole nature of conductivity. A small amount of atoms of a trivalent element (such as indium) is added to a tetravalent semiconductor (such as silicon). Each impurity atom establishes a covalent bond with three neighboring silicon atoms. To establish a bond with the fourth silicon atom, the indium atom does not have a valence electron, so it grabs a valence electron from the covalent bond between neighboring silicon atoms and becomes a negatively charged ion, resulting in the formation of a hole. The impurities that are added in this case are called acceptor impurities.
The conductivity of p-semiconductors is approximately equal to:
Use in radio engineering
Semiconductor diode
A semiconductor diode consists of two types of semiconductors - hole and electron. During contact between these regions, electrons pass from the region with the n-type semiconductor to the region with the p-type semiconductor, which then recombine with holes. As a result, an electric field arises between the two regions, which sets the limit for the division of semiconductors - the so-called p-n junction. As a result, an uncompensated charge of negative ions appears in the region with a p-type semiconductor, and an uncompensated charge of positive ions appears in the region with an n-type semiconductor. The difference between the potentials reaches 0.3-0.6 V.
The relationship between potential difference and impurity concentration is expressed by the following formula:
where is the thermodynamic stress, is the electron concentration, is the hole concentration, is the intrinsic concentration.
In the process of applying a plus voltage to the p-semiconductor and a minus to the n-semiconductor, the external electric field will be directed against the internal electric field of the p-n junction and, with sufficient voltage, the electrons will overcome the p-n junction, and an electric current will appear in the diode circuit (direct conduction). When a minus voltage is applied to an area with a p-type semiconductor and a plus voltage to an area with an n-type semiconductor, a region appears between the two areas that does not have free electric current carriers (reverse conduction). The reverse current of a semiconductor diode is not zero because there are always minority charge carriers in both regions. For these carriers the pn junction will be open.
Thus, the p-n junction exhibits the properties of one-way conductivity, which is caused by applying voltage with different polarities. This property is used to rectify alternating current.
Transistor
A transistor is a semiconductor device that consists of two regions with p- or n-type semiconductors, between which there is a region with an n- or p-type semiconductor. Thus, there are two p-n junction regions in the transistor. The region of the crystal between the two junctions is called the base, and the outer regions are called the emitter and collector. The most commonly used transistor connection circuit is a connection circuit with a common emitter, in which the current propagates through the base and emitter to the collector.
A bipolar transistor is used to amplify electric current.
Types of semiconductors in the periodic table of elements
The table below provides information on a large number of semiconductor elements and their connections, divided into several types:
- single-element semiconductors of group IV of the periodic table of elements,
- complex: two-element A III B V and A II B VI from the third and fifth groups and from the second and sixth groups of elements, respectively.
All types of semiconductors have an interesting dependence of the band gap on the period, namely, as the period increases, the band gap decreases.
| Group | IIB | IIIA | IVA | V.A. | VIA |
| Period | |||||
| 2 | 5 | 6 | 7 | ||
| 3 | 13 | 14 | 15 | 16 | |
| 4 | 30 | 31 | 32 | 33 | 34 |
| 5 | 48 | 49 | 50 | 51 | 52 |
| 6 | 80 |
Physical properties and applications
First of all, it should be said that the physical properties of semiconductors are the most studied in comparison with metals and dielectrics. To a large extent, this is facilitated by a huge number of effects that cannot be observed in either one or another substance, primarily related to the structure of the band structure of semiconductors and the presence of a fairly narrow band gap. Of course, the main incentive for studying semiconductors is the production of semiconductor devices and integrated circuits - this primarily applies to silicon, but also affects other compounds (GaAs, InP, InSb).
Due to the fact that technologists can obtain very pure substances, the question arises about a new standard for Avogadro's number.
Alloying
The bulk properties of a semiconductor can greatly depend on the presence of defects in the crystal structure. And therefore they strive to grow very pure substances, mainly for the electronics industry. Dopants are introduced to control the amount and type of conductivity of the semiconductor. For example, widespread silicon can be doped with an element of the V subgroup of the periodic table of elements - phosphorus, which is a donor, and create n-Si. To obtain silicon with hole type conductivity (p-Si), boron (acceptor) is used. Compensated semiconductors are also created in order to fix the Fermi level in the middle of the bandgap.
Receipt methods
To obtain semiconductor single crystals, various methods of physical and chemical deposition are used. The most precise and expensive tool in the hands of technologists for the growth of single-crystal films is molecular beam epitaxy units, which allow growing a crystal with precision down to a monolayer.
Semiconductor optics
The absorption of light by semiconductors is due to transitions between energy states of the band structure. Given the Pauli exclusion principle, electrons can only move from a filled energy level to an unfilled one. In an intrinsic semiconductor, all states of the valence band are filled, and all states of the conduction band are unfilled, therefore transitions are possible only from the valence band to the conduction band. To make such a transition, the electron must receive energy from light that exceeds the band gap. Photons with lower energy do not cause transitions between the electronic states of the semiconductor, therefore such semiconductors are transparent in the frequency range, where is the band gap and is Planck's constant. This frequency determines the fundamental absorption edge for the semiconductor. For semiconductors, which are often used in electronics (silicon, germanium, gallium arsenide), it lies in the infrared region of the spectrum.
Additional restrictions on the absorption of light by semiconductors are imposed by selection rules, in particular the law of conservation of momentum. The law of conservation of momentum requires that the quasi-momentum of the final state differ from the quasi-momentum of the initial state by the magnitude of the momentum of the absorbed photon. The wave number of the photon, where is the wavelength, is very small compared to the wave vector of the reciprocal lattice of the semiconductor, or, what is the same, the wavelength of the photon in the visible region is much greater than the characteristic interatomic distance in the semiconductor, which leads to the requirement that the quasi-momentum of a finite state during the electronic transition was practically equal to the quasi-momentum of the initial state. At frequencies close to the fundamental absorption edge, this is only possible for direct-gap semiconductors. Optical transitions in semiconductors during which the electron momentum remains almost unchanged are called straight or vertical. The momentum of the final state can differ significantly from the momentum of the initial state if another, third particle, for example, a phonon, is involved in the process of photon absorption. Such transitions are also possible, although less likely. They are called indirect transitions.
Thus, direct-gap semiconductors such as gallium arsenide begin to strongly absorb light when the quantum energy exceeds the bandgap. Such semiconductors are very convenient for use in optoelectronics.
Indirect-gap semiconductors, for example, silicon, absorb in the frequency range of light with a quantum energy slightly greater than the band gap much weaker, only due to indirect transitions, the intensity of which depends on the presence of phonons, and therefore on temperature. The cutoff frequency of direct transitions in silicon is greater than 3 eV, that is, it lies in the ultraviolet region of the spectrum.
When an electron passes from the valence band to the conduction band, free charge carriers appear in the semiconductor, and hence photoconductivity.
At frequencies below the fundamental absorption edge, absorption of light is also possible, which is associated with excitation of excitons, electronic transitions between impurity levels and allowed bands, as well as absorption of light by lattice vibrations and free carriers. Exciton bands are located in the semiconductor slightly below the bottom of the conduction band due to the exciton binding energy. Exciton absorption spectra have a hydrogen-like structure of energy levels. Similarly, impurities, acceptors or donors, create acceptor or donor levels lying in the band gap. They significantly modify the absorption spectrum of the doped semiconductor. If during an indirect gap transition a phonon is absorbed simultaneously with a light quantum, then the energy of the absorbed light quantum may be less by the amount of the phonon energy, which leads to absorption at frequencies slightly lower in energy from the fundamental absorption edge.
List of semiconductors
Semiconductor compounds are divided into several types:
- simple semiconductor materials - the actual chemical elements: boron B, carbon C, germanium Ge, silicon Si, selenium Se, sulfur S, antimony Sb, tellurium Te and iodine I. Germanium, silicon and selenium are widely used independently. The rest are most often used as dopants or as components of complex semiconductor materials;
- to the group complex Semiconductor materials include chemical compounds that have semiconductor properties and include two, three or more chemical elements. Semiconductor materials of this group, consisting of two elements, are called binary, and just as is customary in chemistry, they have the name of the component whose metallic properties are less pronounced. Thus, binary compounds containing arsenic are called arsenides, sulfur - sulfides, tellurium - tellurides, carbon - carbides. Complex semiconductor materials are united by the group number of the Periodic Table of Elements of D.I. Mendeleev, to which the components of the compound belong, and are designated by letters of the Latin alphabet (A is the first element, B is the second, etc.). For example, the binary compound indium phosphide InP is designated A III B V
The following compounds are widely used:
A III B V
- InSb, InAs, InP, GaSb, GaP, AlSb, GaN, InN
- CdSb, ZnSb
- ZnS, ZnSe, ZnTe, CdS, CdTe, HgSe, HgTe, HgS
- PbS, PbSe, PbTe, SnTe, SnS, SnSe, GeS, GeSe
as well as some oxides of lead, tin, germanium, silicon as well as ferrite, amorphous glasses and many other compounds (A I B III C 2 VI, A I B V C 2 VI, A II B IV C 2 V, A II B 2 II C 4 VI, A II B IV C 3 VI).
Based on most of the above binary compounds, it is possible to obtain their solid solutions: (CdTe) x (HgTe) 1-x, (HgTe) x (HgSe) 1-x, (PbTe) x (SnTe) 1-x, (PbSe) x (SnSe) 1-x and others.
Connections A III B V are mainly used for electronic products operating at ultrahigh frequencies
A II B V compounds are used as visible region phosphors, LEDs, Hall sensors, and modulators.
Compounds A III B V, A II B VI and A IV B VI are used in the manufacture of light sources and receivers, indicators and radiation modulators.
Oxide semiconductor compounds are used for the manufacture of photovoltaic cells, rectifiers and high-frequency inductor cores.
| Options | AlSb | GaSb | InSb | AlAs | GaAs | InAs |
|---|---|---|---|---|---|---|
| Melting point, K | 1333 | 998 | 798 | 1873 | 1553 | 1218 |
| lattice constant, | 6,14 | 6,09 | 6,47 | 5,66 | 5,69 | 6,06 |
| Band gap Δ E, eV | 0,52 | 0,7 | 0,18 | 2,2 | 1,32 | 0,35 |
| Dielectric constant ε | 8,4 | 14,0 | 15,9 | - | - | - |
| Mobility, cm²/(V s): | ||||||
| electrons | 50 | 5000 | 60 000 | - | 4000 | 3400 |
| holes | 150 | 1000 | 4000 | - | 400 | 460 |
| Refractive index of light, n | 3,0 | 3,7 | 4,1 | - | 3,2 | 3,2 |
| Linear thermal coefficient extensions, K -1 |
- | 6.9·10 -6 | 5.5·10 -6 | 5.7·10 -6 | 5.3·10 -6 | - |
Various types of semiconductors have become widespread in industry and energy microelectronics. With their help, one energy can be converted into another; without them, many electronic devices will not work normally. There are a large number of types of these elements, depending on the principle of their operation, purpose, material, and design features. In order to understand the mode of action of semiconductors, it is necessary to know their basic physical properties.
Properties and characteristics of semiconductors
The basic electrical properties of semiconductors allow them to be considered as a cross between standard conductors and materials that do not conduct electricity. The semiconductor group includes significantly more different substances than the total number.
Semiconductors made from silicon, germanium, selenium and other materials are widely used in electronics. Their main characteristic is considered to be a pronounced dependence on the influence of temperature. At very low temperatures, comparable to absolute zero, semiconductors acquire the properties of insulators, and as the temperature rises, their resistance decreases while their conductivity increases. The properties of these materials can also change under the influence of light, when a significant increase in photoconductivity occurs.
Semiconductors convert light energy into electricity, unlike conductors, which do not have this property. In addition, the introduction of atoms of certain elements into the semiconductor contributes to an increase in electrical conductivity. All these specific properties allow the use of semiconductor materials in various fields of electronics and electrical engineering.
Types and applications of semiconductors
Due to their qualities, all types of semiconductors are divided into several main groups.
Diodes. They include two crystals made of semiconductors with different conductivities. An electron-hole transition is formed between them. They are produced in various designs, mainly point and flat types. In planar cells, the germanium crystal is alloyed with indium. Point diodes consist of a silicon crystal and a metal needle.

Transistors. They consist of three crystalline semiconductors. Two crystals have the same conductivity, and in the third, the conductivity has the opposite value. They are called collector, base and emitter. In electronics, amplifies electrical signals.
Thyristors. They are elements that convert electricity. They have three electron-hole junctions with gate properties. Their properties allow thyristors to be widely used in automation, computers, and control devices.
How does a semiconductor differ from insulators and conductors?
Semiconductors got their name because they occupy an intermediate position between conductors (metals, electrolytes, coal), which have high electrical conductivity, and insulators (porcelain, mica, rubber, and others), which conduct almost no electric current.
If we compare the specific volume resistance in Ohm × cm for various substances, it turns out that the conductors have: ρ U= 10 -6 - 10 -3 Ohm × cm; resistivity of semiconductors: ρ U= 10 -3 - 10 8 Ohm × cm; and for dielectrics: ρ U= 10 8 - 10 20 Ohm × cm. Semiconductors include: metal oxides - oxides (Al 2 O 3, Cu 2 O, ZnO, TiO 2, VO 2, WO 2, MoO 3); sulfur compounds - sulfides (Cu 2 S, Ag 2 S, ZnS, CdS, HgS); compounds with selenium - selenides; compounds with tellurium - tellurides; some alloys (MgSb 2, ZnSb, Mg 2 Sb, CdSb, AlSb, ClSb); chemical elements - germanium, silicon, tellurium, selenium, boron, carbon, sulfur, phosphorus, arsenic, as well as a large number of complex compounds (galene, carborundum and others).
Figure 1. Germanium
![]()
![]()
Figure 2. Silicon


Figure 3. Tellurium
A complete and extensive study of the properties of semiconductors was carried out by the Soviet scientist A.F. Ioffe and his colleagues.
The electrical properties of semiconductors differ sharply from the properties of conductors and insulators. The electrical conductivity of conductors greatly depends on temperature, illumination, the presence and intensity of the electric field, and the amount of impurities. At ordinary temperatures, semiconductors contain a certain number of free electrons resulting from the breaking of electronic bonds. Semiconductors have two types of conductivity: electron and hole. Charge carriers in semiconductors with electronic conduction are free electrons, and with hole conduction they are bonds devoid of electrons.
Consider the following experiment. Let's take a metal conductor and heat one end of it, then the heated end of the conductor will receive a positive charge. This is due to the movement of electrons from the hot end to the cold end, resulting in a shortage of electrons at the hot end of the conductor (positive charge) and an excess of electrons at the cold end (negative charge). The short-term flow of current through a conductor was caused by the movement of electrons from one end of the conductor to the other. Thus, here we are talking about a conductor with electronic conductivity. However, there are substances that behave differently during such an experiment: the heated edge of such a substance receives a negative charge, and the cold edge receives a positive charge. This is possible if we assume that current transfer is carried out by positive charges.


Figure 4. Bonding between atoms of a substance
  |
| Figure 5. Intrinsic conductivity of semiconductors |
  |
| Figure 6. Electronic conductivity of a semiconductor |
  |
| Figure 7. Hole conductivity of a semiconductor |
Let's get acquainted with another type of conductivity in semiconductors - hole conductivity. In pure semiconductors, all electrons weakly bound to the nuclei participate in electronic bonds. In Figure 4, A the filled bond between the atoms of the substance is conventionally shown. A “hole” is an element of the crystal lattice of a substance that has lost an electron, which corresponds to the appearance of a positive charge (Figure 4, b).
A released bond may be filled again if the “hole” captures an electron from a neighboring bond (Figure 4, V). This will cause the "hole" to move to a new location. In a semiconductor substance under normal conditions, the direction of electron emission and the location of the “hole” formation are chaotic. If a constant voltage is applied to a pure semiconductor, then electrons and “holes” will move (the first against the direction of the field forces, the second in the opposite direction). If the number of “holes” formed is equal to the number of released electrons, then, as is the case with pure semiconductors, the conductivity of semiconductors is low (intrinsic conductivity). The presence of even a small amount of foreign impurities can change the mechanism of electrical conductivity: make it electronic or hole. Let's look at a specific example. Let's take germanium (Ge) as a semiconductor. In a germanium crystal, each atom is bonded to four other atoms. When the temperature increases or as a result of irradiation, the pair bonds of the crystal can be broken. In this case, an equal number of electrons and “holes” are formed (Figure 5).
Let's add arsenic to germanium as an impurity. Such an impurity has a large number of weakly bound electrons. Impurity atoms have their own energy level, located between the energy levels of the free and filled bands, closer to the latter (Figure 6). Such impurities give up their electrons to the free zone and are called donor impurities. The semiconductor will have free electrons, while all bonds will be filled. The semiconductor will have electronic conductivity in the free band.
If now indium, rather than arsenic, is added as an impurity to germanium, the following will happen. Such an impurity has a small number of weakly bound electrons, and the energy level of the impurity is located between the energy levels of the free and filled bands, closer to the free band (Figure 7). Impurities of this kind accept electrons from an adjacent filled zone into their zone and are called acceptor impurities. In the semiconductor there will be unfilled bonds - “holes” in the absence of free electrons. The semiconductor will have hole conductivity in the filled band.
Now the experience of heating a semiconductor will become clear, when the heated end received a negative charge, and the cold end received a positive charge. Under the influence of heat, bonds at the hot end will begin to break down, creating “holes” and free electrons. If the semiconductor contains impurities, then the “holes” will begin to move to the cold end, charging it positively, and the heated end of the semiconductor will become negatively charged.
Concluding our consideration of semiconductors, we draw the following conclusion.
By adding impurities to a semiconductor, one can give it predominant electronic or hole conductivity. Based on this, the following types of semiconductors are obtained. Semiconductors with electronic conductivity are called semiconductors n-type (negative), and with hole conductivity - p-type (positive).
We also invite you to watch educational videos about semiconductors:
List=PL_QCOTUIndSFAbWcR3t0wYp5IORVEHu3I