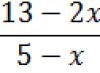Lecture 2. The basic law of radioactive decay and the activity of radionuclides
The rate of decay of radionuclides is different - some decay faster, others slower. An indicator of the rate of radioactive decay is radioactive decay constant, λ [sec-1], which characterizes the probability of the decay of one atom in one second. For each radionuclide, the decay constant has its own value; the larger it is, the faster the nuclei of the substance decay.
The number of decays recorded in a radioactive sample per unit time is called activity (a ), or the radioactivity of the sample. The activity value is directly proportional to the number of atoms N radioactive substance:
a =λ· N , (3.2.1)
Where λ – radioactive decay constant, [sec-1].
Currently, according to the current International System of Units SI, the unit of measurement of radioactivity is becquerel [Bk]. This unit received its name in honor of the French scientist Henri Becquerel, who discovered the phenomenon of natural radioactivity of uranium in 1856. One becquerel equals one decay per second 1 Bk = 1 .
However, the non-system unit of activity is still often used – curie [Ki], introduced by the Curies as a measure of the decay rate of one gram of radium (in which ~3.7 1010 decays per second occur), therefore
1 Ki= 3.7·1010 Bk.
This unit is convenient for assessing the activity of large quantities of radionuclides.
The decrease in radionuclide concentration over time as a result of decay obeys an exponential relationship:
 , (3.2.2)
, (3.2.2)
Where N t– the number of atoms of a radioactive element remaining after time t after the start of observation; N 0 – number of atoms at the initial moment of time ( t =0 ); λ – radioactive decay constant.
The described dependence is called basic law of radioactive decay .
The time during which half of the total amount of radionuclides decays is called half-life T½ . After one half-life, out of 100 radionuclide atoms, only 50 remain (Fig. 2.1). Over the next similar period, only 25 of these 50 atoms remain, and so on.
The relationship between half-life and decay constant is derived from the equation of the fundamental law of radioactive decay:
at t=T½ And
we get https://pandia.ru/text/80/150/images/image006_47.gif" width="67" height="41 src="> Þ ;
https://pandia.ru/text/80/150/images/image009_37.gif" width="76" height="21">;
i.e..gif" width="81" height="41 src=">.
Therefore, the law of radioactive decay can be written as follows:
https://pandia.ru/text/80/150/images/image013_21.gif" width="89" height="39 src=">, (3.2.4)
Where at – drug activity over time t ; a0 – activity of the drug at the initial moment of observation.
It is often necessary to determine the activity of a given amount of any radioactive substance.
Remember that the unit of quantity of a substance is the mole. A mole is the amount of a substance containing the same number of atoms as are contained in 0.012 kg = 12 g of the carbon isotope 12C.
One mole of any substance contains Avogadro's number N.A. atoms:
N.A. = 6.02·1023 atoms.
For simple substances (elements), the mass of one mole numerically corresponds to the atomic mass A element
1mol = A G.
For example: For magnesium: 1 mol 24Mg = 24 g.
For 226Ra: 1 mol 226Ra = 226 g, etc.
Taking into account what has been said in m grams of the substance will be N atoms:
https://pandia.ru/text/80/150/images/image015_20.gif" width="156" height="43 src="> (3.2.6)
Example: Let's calculate the activity of 1 gram of 226Ra, which λ = 1.38·10-11 sec-1.
a= 1.38·10-11·1/226·6.02·1023 = 3.66·1010 Bq.
If a radioactive element is part of a chemical compound, then when determining the activity of the drug it is necessary to take into account its formula. Taking into account the composition of the substance, the mass fraction is determined χ radionuclide in a substance, which is determined by the ratio:
https://pandia.ru/text/80/150/images/image017_17.gif" width="118" height="41 src=">
Example of problem solution
Condition:
Activity A0 radioactive element 32P per day of observation is 1000 Bk. Determine the activity and number of atoms of this element after a week. Half life T½ 32P = 14.3 days.
Solution:
a) Let’s find the activity of phosphorus-32 after 7 days:
https://pandia.ru/text/80/150/images/image019_16.gif" width="57" height="41 src=">
Answer: after a week, the activity of the drug 32P will be 712 Bk, and the number of atoms of the radioactive isotope 32P is 127.14·106 atoms.
Security questions
1) What is the activity of a radionuclide?
2) Name the units of radioactivity and the relationship between them.
3) What is the radioactive decay constant?
4) Define the basic law of radioactive decay.
5) What is half-life?
6) What is the relationship between activity and mass of a radionuclide? Write the formula.
Tasks
1. Calculate activity 1 G 226Ra. T½ = 1602 years.
2. Calculate activity 1 G 60Co. T½ = 5.3 years.
3. One M-47 tank shell contains 4.3 kg 238U. Т½ = 2.5·109 years. Determine the activity of the projectile.
4. Calculate the activity of 137Cs after 10 years, if at the initial moment of observation it is equal to 1000 Bk. T½ = 30 years.
5. Calculate the activity of 90Sr a year ago if it is currently equal to 500 Bk. T½ = 29 years.
6. What kind of activity will 1 create? kg radioisotope 131I, T½ = 8.1 days?
7. Using reference data, determine activity 1 G 238U. Т½ = 2.5·109 years.
Using reference data, determine activity 1 G 232Th, Т½ = 1.4·1010 years.
8. Calculate the activity of the compound: 239Pu316O8.
9. Calculate the mass of a radionuclide with an activity of 1 Ki:
9.1. 131I, T1/2=8.1 days;
9.2. 90Sr, T1/2=29 years;
9.3. 137Cs, Т1/2=30 years;
9.4. 239Pu, Т1/2=2.4·104 years.
10. Determine mass 1 mCi radioactive carbon isotope 14C, T½ = 5560 years.
11. It is necessary to prepare a radioactive preparation of phosphorus 32P. After what period of time will 3% of the drug remain? Т½ = 14.29 days.
12. The natural potassium mixture contains 0.012% of the 40K radioactive isotope.
1) Determine the mass of natural potassium, which contains 1 Ki 40K. Т½ = 1.39·109 years = 4.4·1018 sec.
2) Calculate the radioactivity of the soil using 40K, if it is known that the potassium content in the soil sample is 14 kg/t.
13. How many half-lives are required for the initial activity of a radioisotope to decrease to 0.001%?
14. To determine the effect of 238U on plants, seeds were soaked in 100 ml solution UO2(NO3)2 6H2O, in which the mass of radioactive salt was 6 G. Determine the activity and specific activity of 238U in solution. Т½ = 4.5·109 years.
15. Identify activity 1 grams 232Th, Т½ = 1.4·1010 years.
16. Determine mass 1 Ki 137Cs, Т1/2=30 years.
17. The ratio between the content of stable and radioactive isotopes of potassium in nature is a constant value. The 40K content is 0.01%. Calculate the radioactivity of the soil using 40K, if it is known that the potassium content in the soil sample is 14 kg/t.
18. Lithogenic radioactivity of the environment is formed mainly due to three main natural radionuclides: 40K, 238U, 232Th. The proportion of radioactive isotopes in the natural sum of isotopes is 0.01, 99.3, ~100, respectively. Calculate radioactivity 1 T soil, if it is known that the relative content of potassium in the soil sample is 13600 g/t, uranium – 1·10-4 g/t, thorium – 6·10-4 g/t.
19. 23,200 were found in the shells of bivalve mollusks Bq/kg 90Sr. Determine the activity of samples after 10, 30, 50, 100 years.
20. The main pollution of closed reservoirs in the Chernobyl zone took place in the first year after the accident at the nuclear power plant. In the bottom sediments of the lake. Azbuchin in 1999 discovered 137Cs with a specific activity of 1.1·10 Bq/m2. Determine the concentration (activity) of fallen 137Cs per m2 of bottom sediments as of 1986-1987. (12 years ago).
21. 241Am (T½ = 4.32·102 years) is formed from 241Pu (T½ = 14.4 years) and is an active geochemical migrant. Using reference materials, calculate with an accuracy of 1% the decrease in the activity of plutonium-241 over time, in which year after the Chernobyl disaster the formation of 241Am in the environment will be maximum.
22. Calculate the activity of 241Am in the emissions of the Chernobyl reactor as of April
2015, provided that in April 1986 the activity of 241Am was 3.82 1012 Bk,Т½ = 4.32·102 years.
23. 390 were found in soil samples nCi/kg 137Cs. Calculate the activity of samples after 10, 30, 50, 100 years.
24. Average concentration of lake bed pollution. Glubokoye, located in the Chernobyl exclusion zone, is 6.3 104 Bk 241Am and 7.4·104 238+239+240Pu per 1 m2. Calculate in what year these data were obtained.
Radioactive decay of atomic nuclei occurs spontaneously and leads to a continuous decrease in the number of atoms of the original radioactive isotope and the accumulation of atoms of the decay product.
The rate at which radionuclides decay is determined only by the degree of instability of their nuclei and does not depend on any factors that usually affect the rate of physical and chemical processes (pressure, temperature, chemical form of the substance, etc.). The decay of each individual atom is a completely random event, probabilistic and independent of the behavior of other nuclei. However, if there is a sufficiently large number of radioactive atoms in the system, a general pattern appears that the number of atoms of a given radioactive isotope decaying per unit time always constitutes a certain fraction, characteristic of a given isotope, of the total number of atoms that have not yet decayed. The number of DUU atoms that have undergone decay in a short period of time D/ is proportional to the total number of undecayed radioactive atoms DU and the value of the DL interval. This law can be mathematically represented as the ratio:
-AN = X ? N? D/.
The minus sign indicates that the number of radioactive atoms N decreases. Proportionality factor X is called decay constant and is a constant characteristic of a given radioactive isotope. The law of radioactive decay is usually written as a differential equation:
So, law of radioactive decay can be formulated as follows: per unit time, the same part of the available nuclei of a radioactive substance always decays.
Decay constant X has the dimension of inverse time (1/s or s -1). The more X, the faster the decay of radioactive atoms occurs, i.e. X characterizes the relative decay rate for each radioactive isotope or the probability of the decay of an atomic nucleus in 1 s. The decay constant is the fraction of atoms decaying per unit time, an indicator of the instability of a radionuclide.
Value - absolute rate of radioactive decay -
called activity. Radionuclide activity (A) - This is the number of atomic decays occurring per unit time. It depends on the number of radioactive atoms at a given time (AND) and on the degree of their instability:
A=Y ( X.
The SI unit of activity is becquerel(Bq); 1 Bq - activity at which one nuclear transformation occurs per second, regardless of the type of decay. Sometimes an off-system unit of measurement of activity is used - the curie (Ci): 1Ci = 3.7-10 10 Bq (the number of decays of atoms in 1 g 226 Ra in 1 s).
Since activity depends on the number of radioactive atoms, this value serves as a quantitative measure of the content of radionuclides in the sample being studied.
In practice, it is more convenient to use the integral form of the law of radioactive decay, which has the following form:
where УУ 0 - number of radioactive atoms at the initial moment of time / = 0; - the number of radioactive atoms remaining at the moment
time /; X- decay constant.
To characterize radioactive decay, often instead of a decay constant X They use another quantity derived from it - the half-life. Half-life (T]/2)- this is the period of time during which half of the initial number of radioactive atoms decays.
Substituting the values G = into the law of radioactive decay T 1/2 And AND (= Af/2, we get:
VU 0 /2 = # 0 e~ xt og-
1 /2 = e~ xt "/2 -, A e xt "/ 2 = 2 or HT 1/2 = 1p2.
The half-life and decay constant are related by the following relationship:
T x/2=1п2 А = 0.693 /X.
Using this relationship, the law of radioactive decay can be presented in another form:
TU, = УУ 0 e Apg, "t t
N = And 0? e-°’ t - ( / t 02.
From this formula it follows that the longer the half-life, the slower the radioactive decay occurs. Half-lives characterize the degree of stability of the radioactive nucleus and vary widely for different isotopes - from fractions of a second to billions of years (see appendices). Depending on their half-life, radionuclides are conventionally divided into long-lived and short-lived.
The half-life, along with the type of decay and radiation energy, is the most important characteristic of any radionuclide.
In Fig. Figure 3.12 shows the decay curve of a radioactive isotope. The horizontal axis represents time (in half-lives), and the vertical axis represents the number of radioactive atoms (or activity, since it is proportional to the number of radioactive atoms).
The curve is exponent and asymptotically approaches the time axis without ever crossing it. After a period of time equal to one half-life (Г 1/2), the number of radioactive atoms decreases by 2 times; after two half-lives (2Г 1/2), the number of remaining atoms again decreases by half, i.e. 4 times from their initial number, after 3 7" 1/2 - 8 times, after
4G 1/2 - 16 times, through T half-lives Г ]/2 - in 2 t once.
Theoretically, the population of atoms with unstable nuclei will decrease to infinity. However, from a practical point of view, a certain limit should be designated when all radioactive nuclides have decayed. It is believed that this requires a period of time of 107^, 2, after which less than 0.1% of radioactive atoms will remain of the original amount. Thus, if we take into account only physical decay, it will take 290 and 300 years, respectively, to completely cleanse the biosphere of 90 Bg (= 29 years) and |37 Cz (T|/ 2 = 30 years) of Chernobyl origin.
Radioactive balance. If, during the decay of a radioactive isotope (parent), a new radioactive isotope (daughter) is formed, then they are said to be genetically related to each other and form radioactive family(row).
Let us consider the case of genetically related radionuclides, of which the parent is long-lived and the daughter is short-lived. An example is strontium 90 5g, which is converted by (3-decay ( T /2 = 64 h) and turns into a stable zirconium nuclide ^Ъх(see Fig. 3.7). Since 90 U decays much faster than 90 5g, after some time there will come a moment when the amount of decaying 90 8g at any moment will be equal to the amount of decaying 90 U. In other words, the activity of the parent 90 8g (D,) will be equal to the activity of the daughter 90 U (L 2). When this happens, 90 V is considered to be in secular equilibrium with its parent radionuclide 90 8g. In this case the relation holds:
A 1 = L 2 or X 1? = X 2?УУ 2 or: Г 1/2(1) = УУ 2: Г 1/2(2) .
From the above relationship it follows that the greater the probability of decay of a radionuclide (To) and, accordingly, a shorter half-life (T ]/2), the less its atoms are contained in a mixture of two isotopes (AO-
Establishing such equilibrium requires a time of approximately 7T ]/2 daughter radionuclide. Under conditions of secular equilibrium, the total activity of a mixture of nuclides is twice as high as the activity of the parent nuclide at a given point in time. For example, if at the initial time the drug contains only 90 8g, then after 7T /2 the longest-lived member of the family (except for the ancestor of the series), a secular equilibrium is established, and the decay rates of all members of the radioactive family become the same. Considering that the half-lives for each member of the family are different, the relative amounts (including mass) of nuclides in equilibrium are also different. The less T = 1[Bq] – becquerel
1Mdisp/s =10 6 disp/s = 1 [Rd] - rutherford
B. Non-system units of measurement.
[Ki] - curie(corresponds to the activity of 1g of radium).
1[Ci] = 3.7 10 10 [disp/s]- 1 g of radium decays in 1 s 3.7 10 10 radioactive nuclei.
Types of activity:(slide 45)
1. Specific is the activity per unit mass of a substance.
A beat = dA/dm [Bq/kg].
It is used to characterize powdery and gaseous substances.
2. Volumetric- is the activity per unit volume of a substance or medium.
A about = dA/dV [Bq/m 3 ]
It is used to characterize liquid substances.
In practice, the decrease in activity is measured using special radiometric instruments. For example, knowing the activity of the drug and the product formed during the decay of 1 nucleus, you can calculate how many particles of each type are emitted by the drug in 1 second.
If “n” neutrons are produced during nuclear fission, then a flux of “N” neutrons is emitted in 1 s. N = n A.
©2015-2019 site
All rights belong to their authors. This site does not claim authorship, but provides free use.
Page creation date: 2016-08-08
The term “radioactivity”, which gets its name from the Latin words “radio” - “radiate” and “activus” - “active”, means the spontaneous transformation of atomic nuclei, accompanied by the emission of gamma radiation, elementary particles or lighter nuclei. All types of radioactive transformations known to science are based on the fundamental (strong and weak) interactions of the particles that make up the atom. A previously unknown type of penetrating radiation emitted by uranium was discovered in 1896 by the French scientist Antoine Henri Becquerel, and the concept of “radioactivity” was introduced into widespread use at the beginning of the 20th century by Marie Curie, who, by studying invisible rays emitted by some minerals, was able to isolate pure radioactive element - radium.
Differences between radioactive transformations and chemical reactions
The main feature of radioactive transformations is that they occur spontaneously, while chemical reactions in any case require some external influences. In addition, radioactive transformations occur continuously and are always accompanied by the release of a certain amount of energy, which depends on the strength of interaction of atomic particles with each other. The rate of reactions inside atoms is not affected by temperature, the presence of electric and magnetic fields, the use of the most effective chemical catalyst, pressure, or the state of aggregation of a substance. Radioactive transformations do not depend on any external factor and can neither be accelerated nor slowed down.
Law of Radioactive Decay
The rate of radioactive decay, as well as its dependence on the number of atoms and time, is expressed in the Law of Radioactive Decay, discovered by Ernest Rutherford and Frederick Soddy in 1903. In order to come to certain conclusions, which were subsequently reflected in the new law, scientists conducted the following experiment: they separated one of the radioactive products and studied its independent activity separately from the radioactivity of the substance from which it was isolated. As a result, it was discovered that the activity of any radioactive products, regardless of the chemical element, decreases exponentially over time. Based on this, scientists concluded that the rate of radioactive transformation is always proportional to the number of systems that have not yet undergone transformation.
The formula for the Law of Radioactive Decay is as follows:
according to which the number of decays −dN occurring over a period of time dt (a very short interval) is proportional to the number of atoms N. In the formula of the Law of Radioactive Decay there is another important quantity - the decay constant (or the reciprocal of the half-life) λ, which characterizes the probability of nuclear decay per unit of time.
What chemical elements are radioactive?
The instability of atoms of chemical elements is rather an exception than a pattern; for the most part they are stable and do not change over time. However, there is a certain group of chemical elements whose atoms are more susceptible to decay than others and, when decaying, emit energy and also release new particles. The most common chemical elements are radium, uranium and plutonium, which have the ability to transform into other elements with simpler atoms (for example, uranium transforms into lead).
1. Radioactivity. The basic law of radioactive decay. Activity.
2. Main types of radioactive decay.
3. Quantitative characteristics of the interaction of ionizing radiation with matter.
4. Natural and artificial radioactivity. Radioactive series.
5. Use of radionuclides in medicine.
6. Accelerators of charged particles and their use in medicine.
7. Biophysical basis of the action of ionizing radiation.
8. Basic concepts and formulas.
9. Tasks.
The interest of doctors in natural and artificial radioactivity is due to the following.
Firstly, all living things are constantly exposed to natural background radiation, which consists of cosmic radiation, radiation from radioactive elements located in the surface layers of the earth’s crust, and radiation from elements entering the body of animals along with air and food.
Secondly, radioactive radiation is used in medicine itself for diagnostic and therapeutic purposes.
33.1. Radioactivity. The basic law of radioactive decay. Activity
The phenomenon of radioactivity was discovered in 1896 by A. Becquerel, who observed the spontaneous emission of unknown radiation from uranium salts. Soon E. Rutherford and the Curies established that during radioactive decay He nuclei (α-particles), electrons (β-particles) and hard electromagnetic radiation (γ-rays) are emitted.
In 1934, decay with the emission of positrons (β + -decay) was discovered, and in 1940, a new type of radioactivity was discovered - spontaneous fission of nuclei: a fissioning nucleus falls apart into two fragments of comparable mass with the simultaneous emission of neutrons and γ -quanta. Proton radioactivity of nuclei was observed in 1982.
Radioactivity - the ability of some atomic nuclei to spontaneously (spontaneously) transform into other nuclei with the emission of particles.
Atomic nuclei consist of protons and neutrons, which have a general name - nucleons. The number of protons in the nucleus determines the chemical properties of the atom and is denoted by Z (this is serial number chemical element). The number of nucleons in a nucleus is called mass number and denote A. Nuclei with the same atomic number and different mass numbers are called isotopes. All isotopes of one chemical element have identical chemical properties. The physical properties of isotopes can vary greatly. To designate isotopes, use the symbol of a chemical element with two indices: A Z X. The lower index is the serial number, the upper index is the mass number. Often the subscript is omitted because it is indicated by the element's symbol itself. For example, they write 14 C instead of 14 6 C.
The ability of a nucleus to decay depends on its composition. The same element can have both stable and radioactive isotopes. For example, the carbon isotope 12 C is stable, but the isotope 14 C is radioactive.
Radioactive decay is a statistical phenomenon. The ability of an isotope to decay characterizes decay constantλ.
Decay constant- the probability that the nucleus of a given isotope will decay per unit time.
The probability of nuclear decay in a short time dt is found by the formula
Taking into account formula (33.1), we obtain an expression that determines the number of decayed nuclei:
 Formula (33.3) is called the main law of radioactive decay.
Formula (33.3) is called the main law of radioactive decay.
The number of radioactive nuclei decreases with time according to an exponential law.
In practice, instead decay constantλ another quantity is often used, called half-life.
Half life(T) - time during which it decays half radioactive nuclei.
The law of radioactive decay using half-life is written as follows:
The graph of dependence (33.4) is shown in Fig. 33.1.
The half-life can be very long or very short (from fractions of a second to many billions of years). In table Figure 33.1 shows the half-lives for some elements.
 Rice. 33.1. Decrease in the number of nuclei of the original substance during radioactive decay
Rice. 33.1. Decrease in the number of nuclei of the original substance during radioactive decay
Table 33.1. Half-lives for some elements
 For evaluation degree of radioactivity isotope use a special quantity called activity.
For evaluation degree of radioactivity isotope use a special quantity called activity.
Activity - number of nuclei of a radioactive drug decaying per unit time:
The SI unit of activity is becquerel(Bq), 1 Bq corresponds to one decay event per second. In practice, more
childish non-systemic unit of activity - curie(Ci), equal to the activity of 1 g 226 Ra: 1 Ci = 3.7x10 10 Bq.
Over time, activity decreases in the same way as the number of undecayed nuclei decreases:
 33.2. Main types of radioactive decay
33.2. Main types of radioactive decay
In the process of studying the phenomenon of radioactivity, 3 types of rays emitted by radioactive nuclei were discovered, which were called α-, β- and γ-rays. It was later discovered that α and β particles are products of two different types of radioactive decay, and γ rays are a byproduct of these processes. In addition, γ-rays also accompany more complex nuclear transformations, which are not considered here.
Alpha decay consists in the spontaneous transformation of nuclei with the emissionα -particles (helium nuclei).
The α-decay scheme is written as
 where X, Y are the symbols of the mother and daughter nuclei, respectively. When writing α-decay, you can write “He” instead of “α”.
where X, Y are the symbols of the mother and daughter nuclei, respectively. When writing α-decay, you can write “He” instead of “α”.
During this decay, the atomic number Z of the element decreases by 2, and the mass number A decreases by 4.
During α-decay, the daughter nucleus, as a rule, is formed in an excited state and, upon transition to the ground state, emits a γ-quantum. The general property of complex microobjects is that they have discrete a set of energy states. This also applies to kernels. Therefore, γ-radiation from excited nuclei has a discrete spectrum. Consequently, the energy spectrum of α-particles is discrete.
The energy of emitted α-particles for almost all α-active isotopes lies in the range of 4-9 MeV.
Beta decay consists in the spontaneous transformation of nuclei with the emission of electrons (or positrons).
It has been established that β-decay is always accompanied by the emission of a neutral particle - a neutrino (or antineutrino). This particle practically does not interact with matter and will not be considered further. The energy released during beta decay is distributed randomly between the beta particle and the neutrino. Therefore, the energy spectrum of β-radiation is continuous (Fig. 33.2).
 Rice. 33.2. Energy spectrum of β-decay
Rice. 33.2. Energy spectrum of β-decay
There are two types of β decay.
1. Electronicβ - -decay consists of the transformation of one nuclear neutron into a proton and an electron. In this case, another particle ν" appears - an antineutrino:
 An electron and an antineutrino fly out from the nucleus. The electron β - decay scheme is written in the form
An electron and an antineutrino fly out from the nucleus. The electron β - decay scheme is written in the form
 During electronic β-decay, the order number of the Z element increases by 1, but the mass number A does not change.
During electronic β-decay, the order number of the Z element increases by 1, but the mass number A does not change.
The energy of β-particles lies in the range of 0.002-2.3 MeV.
2. Positronicβ + -decay involves the transformation of one nuclear proton into a neutron and a positron. In this case, another particle ν appears - a neutrino:
 Electron capture itself does not produce ionizing particles, but it does accompanied by X-ray radiation. This radiation occurs when the space vacated by the absorption of an internal electron is filled by an electron from the outer orbit.
Electron capture itself does not produce ionizing particles, but it does accompanied by X-ray radiation. This radiation occurs when the space vacated by the absorption of an internal electron is filled by an electron from the outer orbit.
Gamma radiation has an electromagnetic nature and represents photons with a wavelengthλ ≤ 10 -10 m.
Gamma radiation is not an independent type of radioactive decay. Radiation of this type almost always accompanies not only α-decay and β-decay, but also more complex nuclear reactions. It is not deflected by electric and magnetic fields, has a relatively weak ionizing and very high penetrating ability.
33.3. Quantitative characteristics of the interaction of ionizing radiation with matter
The impact of radioactive radiation on living organisms is associated with ionization, which it causes in tissues. The ability of a particle to ionize depends on both its type and its energy. As a particle moves deeper into matter, it loses its energy. This process is called ionization inhibition.
To quantitatively characterize the interaction of a charged particle with matter, several quantities are used:
 Once the particle's energy drops below the ionization energy, its ionizing effect ceases.
Once the particle's energy drops below the ionization energy, its ionizing effect ceases.
Average linear mileage(R) of a charged ionizing particle - the path traveled by it in a substance before losing its ionizing ability.
Let us consider some characteristic features of the interaction of various types of radiation with matter.
Alpha radiation
The alpha particle practically does not deviate from the initial direction of its movement, since its mass is many times greater
 Rice. 33.3. Dependence of linear ionization density on the path traveled by an α-particle in the medium
Rice. 33.3. Dependence of linear ionization density on the path traveled by an α-particle in the medium
the mass of the electron with which it interacts. As it penetrates deep into the substance, the ionization density first increases, and when completion of the run (x = R) drops sharply to zero (Fig. 33.3). This is explained by the fact that as the speed of movement decreases, the time it spends near a molecule (atom) of the medium increases. The probability of ionization increases in this case. After the energy of the α particle becomes comparable to the energy of molecular thermal motion, it captures two electrons in the substance and turns into a helium atom.
Electrons formed during the ionization process, as a rule, move away from the α-particle track and cause secondary ionization.
Characteristics of the interaction of α-particles with water and soft tissues are presented in Table. 33.2.
Table 33.2. Dependence of the characteristics of interaction with matter on the energy of α-particles
 Beta radiation
Beta radiation
For movement β -particles in matter are characterized by a curvilinear unpredictable trajectory. This is due to the equality of the masses of interacting particles.
Interaction Characteristics β -particles with water and soft tissues are presented in table. 33.3.
Table 33.3. Dependence of the characteristics of interaction with matter on the energy of β-particles
 Like α particles, the ionization ability of β particles increases with decreasing energy.
Like α particles, the ionization ability of β particles increases with decreasing energy.
Gamma radiation
Absorption γ -radiation by matter obeys an exponential law similar to the law of absorption of X-ray radiation:
The main processes responsible for absorption γ -radiation are the photoelectric effect and Compton scattering. This produces a relatively small number of free electrons (primary ionization), which have very high energy. They cause processes of secondary ionization, which is incomparably higher than the primary one.
33.4. Natural and artificial
radioactivity. Radioactive series
Terms natural And artificial radioactivity are conditional.
Natural called the radioactivity of isotopes existing in nature, or the radioactivity of isotopes formed as a result of natural processes.
For example, the radioactivity of uranium is natural. The radioactivity of carbon 14 C, which is formed in the upper layers of the atmosphere under the influence of solar radiation, is also natural.
Artificial called radioactivity of isotopes that arise as a result of human activity.
This is the radioactivity of all isotopes produced in particle accelerators. This also includes the radioactivity of soil, water and air that occurs during an atomic explosion.
Natural radioactivity
In the initial period of studying radioactivity, researchers could only use natural radionuclides (radioactive isotopes) contained in earth rocks in sufficiently large quantities: 232 Th, 235 U, 238 U. Three radioactive series begin with these radionuclides, ending with stable isotopes Pb. Subsequently, a series was discovered starting from 237 Np, with the final stable nucleus 209 Bi. In Fig. Figure 33.4 shows the row starting with 238 U.
 Rice. 33.4. Uranium-radium series
Rice. 33.4. Uranium-radium series
Elements of this series are the main source of internal human radiation. For example, 210 Pb and 210 Po enter the body with food - they are concentrated in fish and shellfish. Both of these isotopes accumulate in lichens and are therefore present in reindeer meat. The most significant of all natural sources of radiation is 222 Rn - a heavy inert gas resulting from the decay of 226 Ra. It accounts for about half the dose of natural radiation received by humans. Formed in the earth's crust, this gas seeps into the atmosphere and enters water (it is highly soluble).
The radioactive isotope of potassium 40 K is constantly present in the earth's crust, which is part of natural potassium (0.0119%). From the soil, this element enters through the root system of plants and with plant foods (cereals, fresh vegetables and fruits, mushrooms) into the body.
Another source of natural radiation is cosmic radiation (15%). Its intensity increases in mountainous areas due to a decrease in the protective effect of the atmosphere. Sources of natural background radiation are listed in Table. 33.4.
Table 33.4. Component of natural radioactive background
 33.5. Use of radionuclides in medicine
33.5. Use of radionuclides in medicine
Radionuclides are called radioactive isotopes of chemical elements with a short half-life. Such isotopes do not exist in nature, so they are obtained artificially. In modern medicine, radionuclides are widely used for diagnostic and therapeutic purposes.
Diagnostic Application based on the selective accumulation of certain chemical elements by individual organs. Iodine, for example, is concentrated in the thyroid gland, and calcium in the bones.
The introduction of radioisotopes of these elements into the body makes it possible to detect areas of their concentration by radioactive radiation and thus obtain important diagnostic information. This diagnostic method is called by the labeled atom method.
Therapeutic Use radionuclides is based on the destructive effect of ionizing radiation on tumor cells.
1. Gamma therapy- use of high-energy γ-radiation (60 Co source) to destroy deep-lying tumors. To prevent superficial tissues and organs from being subjected to harmful effects, exposure to ionizing radiation is carried out in different sessions in different directions.
2. Alpha therapy- therapeutic use of α-particles. These particles have a significant linear ionization density and are absorbed by even a small layer of air. Therefore therapeutic
The use of alpha rays is possible through direct contact with the surface of the organ or when administered internally (using a needle). For surface exposure, radon therapy (222 Rn) is used: exposure to the skin (baths), digestive organs (drinking), and respiratory organs (inhalation).
In some cases, medicinal use α -particles is associated with the use of neutron flux. With this method, elements are first introduced into the tissue (tumor), the nuclei of which, under the influence of neutrons, emit α -particles. After this, the diseased organ is irradiated with a stream of neutrons. In this way α -particles are formed directly inside the organ on which they should have a destructive effect.
Table 33.5 shows the characteristics of some radionuclides used in medicine.
Table 33.5. Characteristics of isotopes
 33.6. Charged particle accelerators and their use in medicine
33.6. Charged particle accelerators and their use in medicine
Accelerator- an installation in which, under the influence of electric and magnetic fields, directed beams of charged particles with high energy (from hundreds of keV to hundreds of GeV) are produced.
Accelerators create narrow beams of particles with a given energy and small cross section. This allows you to provide directed impact on irradiated objects.
Use of accelerators in medicine
Electron and proton accelerators are used in medicine for radiation therapy and diagnostics. In this case, both the accelerated particles themselves and the accompanying X-ray radiation are used.
Bremsstrahlung X-rays are obtained by directing a beam of particles to a special target, which is the source of X-rays. This radiation differs from the X-ray tube by significantly higher quantum energy.
Synchrotron X-rays occurs during the acceleration of electrons in ring accelerators - synchrotrons. Such radiation has a high degree of directionality.
The direct effect of fast particles is associated with their high penetrating ability. Such particles pass through superficial tissues without causing serious damage and have an ionizing effect at the end of their journey. By selecting the appropriate particle energy, it is possible to destroy tumors at a given depth.
The areas of application of accelerators in medicine are shown in Table. 33.6.
Table 33.6. Application of accelerators in therapy and diagnostics
 33.7. Biophysical basis of the action of ionizing radiation
33.7. Biophysical basis of the action of ionizing radiation
As noted above, the impact of radioactive radiation on biological systems is associated with ionization of molecules. The process of interaction of radiation with cells can be divided into three successive stages (stages).
1. Physical stage consists of energy transfer radiation to molecules of a biological system, resulting in their ionization and excitation. The duration of this stage is 10 -16 -10 -13 s.
2. Physico-chemical the stage consists of various types of reactions leading to the redistribution of excess energy of excited molecules and ions. As a result, highly active
products: radicals and new ions with a wide range of chemical properties.
The duration of this stage is 10 -13 -10 -10 s.
3. Chemical stage - this is the interaction of radicals and ions with each other and with surrounding molecules. At this stage, structural damage of various types is formed, leading to changes in biological properties: the structure and functions of membranes are disrupted; lesions occur in DNA and RNA molecules.
The duration of the chemical stage is 10 -6 -10 -3 s.
4. Biological stage. At this stage, damage to molecules and subcellular structures leads to various functional disorders, to premature cell death as a result of the action of apoptotic mechanisms or due to necrosis. Damage received at the biological stage can be inherited.
The duration of the biological stage is from several minutes to tens of years.
Let us note the general patterns of the biological stage:
Large disturbances with low absorbed energy (a lethal dose of radiation for humans causes the body to heat up by only 0.001°C);
Effect on subsequent generations through the hereditary apparatus of the cell;
Characterized by a hidden, latent period;
Different parts of cells have different sensitivity to radiation;
First of all, dividing cells are affected, which is especially dangerous for a child’s body;
Detrimental effect on tissues of an adult organism in which there is division;
Similarity of radiation changes with the pathology of early aging.
33.8. Basic concepts and formulas
 Continuation of the table
Continuation of the table
 33.9. Tasks
33.9. Tasks
1. What is the activity of the drug if 10,000 nuclei of this substance decay within 10 minutes?
 4.
The age of ancient wood samples can be approximately determined by the specific mass activity of the 14 6 C isotope in them. How many years ago was the tree cut down that was used to make an object, if the specific mass activity of carbon in it is 75% of the specific mass activity of the growing tree? The half-life of radon is T = 5570 years.
4.
The age of ancient wood samples can be approximately determined by the specific mass activity of the 14 6 C isotope in them. How many years ago was the tree cut down that was used to make an object, if the specific mass activity of carbon in it is 75% of the specific mass activity of the growing tree? The half-life of radon is T = 5570 years.
 9.
After the Chernobyl accident, in some places soil contamination with radioactive cesium-137 was at the level of 45 Ci/km 2 .
9.
After the Chernobyl accident, in some places soil contamination with radioactive cesium-137 was at the level of 45 Ci/km 2 .
After how many years will activity in these places decrease to a relatively safe level of 5 Ci/km 2? The half-life of cesium-137 is T = 30 years.
 10.
The permissible activity of iodine-131 in the human thyroid gland should be no more than 5 nCi. In some people who were in the Chernobyl disaster zone, the activity of iodine-131 reached 800 nCi. After how many days did activity decrease to normal? The half-life of iodine-131 is 8 days.
10.
The permissible activity of iodine-131 in the human thyroid gland should be no more than 5 nCi. In some people who were in the Chernobyl disaster zone, the activity of iodine-131 reached 800 nCi. After how many days did activity decrease to normal? The half-life of iodine-131 is 8 days.
 11.
To determine the blood volume of an animal, the following method is used. A small volume of blood is taken from the animal, red blood cells are separated from the plasma and placed in a solution with radioactive phosphorus, which is assimilated by the red blood cells. The labeled red blood cells are reintroduced into the animal's circulatory system, and after some time the activity of the blood sample is determined.
11.
To determine the blood volume of an animal, the following method is used. A small volume of blood is taken from the animal, red blood cells are separated from the plasma and placed in a solution with radioactive phosphorus, which is assimilated by the red blood cells. The labeled red blood cells are reintroduced into the animal's circulatory system, and after some time the activity of the blood sample is determined.
ΔV = 1 ml of such a solution was injected into the blood of some animal. The initial activity of this volume was equal to A 0 = 7000 Bq. The activity of 1 ml of blood taken from the vein of an animal a day later was equal to 38 pulses per minute. Determine the animal’s blood volume if the half-life of radioactive phosphorus is T = 14.3 days.