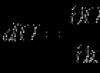Chain reaction
Chain reaction- a chemical and nuclear reaction in which the appearance of an active particle (a free radical or atom in a chemical process, a neutron in a nuclear process) causes a large number (chain) of successive transformations of inactive molecules or nuclei. Free radicals and many atoms, unlike molecules, have free unsaturated valences (unpaired electron), which leads to their interaction with the original molecules. When a free radical (R) collides with a molecule, one of the valence bonds of the latter is broken and, thus, as a result of the reaction, a new free radical is formed, which, in turn, reacts with another molecule - a chain reaction occurs.
Chain reactions in chemistry include the processes of oxidation (combustion, explosion), cracking, polymerization and others, which are widely used in the chemical and oil industries.
Wikimedia Foundation. 2010.
See what “Chain reaction” is in other dictionaries:
CHAIN REACTION, a self-sustaining process of nuclear fission, in which one reaction leads to the beginning of a second, a second to a third, and so on. For the reaction to begin, critical conditions are required, that is, a mass of material capable of splitting... ... Scientific and technical encyclopedic dictionary
chain reaction- Any biological (or chemical-physical) process composed of a series of interconnected processes, where the product (or energy) of each stage is a participant in the next stage, which leads to the maintenance and (or) acceleration of the chain... ... Technical Translator's Guide
chain reaction- 1) A reaction that causes a large number of transformations of the molecules of the original substance. 2) Self-sustaining reaction of fission of atomic nuclei of heavy elements under the influence of neutrons. 3) decomposition About a series of actions, states, etc., in which one or one... ... Dictionary of many expressions
Chain reaction Any biological (or chemical-physical) process composed of a series of interrelated processes, where the product (or energy) of each stage is a participant in the next stage, which leads to the maintenance and (or) ... ... Molecular biology and genetics. Explanatory dictionary.
chain reaction- grandininė reakcija statusas T sritis chemija apibrėžtis Cheminė ar branduolinė reakcija, kurios aktyvusis centras sukelia ilgą kitimų grandinę. atitikmenys: engl. chain reaction rus. chain reaction... Chemijos terminų aiškinamasis žodynas
chain reaction- grandininė reakcija statusas T sritis fizika atitikmenys: engl. chain reaction vok. Kettenkernreaktion, f; Kettenreaktion, f rus. chain reaction, f pranc. réaction en chaîne, f … Fizikos terminų žodynas
Razg. About the ongoing, uncontrolled process of involving someone or something. what? BMS 1998, 489; BTS, 1462… Large dictionary of Russian sayings
Chain reaction scientific concept. And also “Chain Reaction” is the name of several feature films: “Chain Reaction” is a 1962 USSR film. "Chain Reaction" is a 1963 French crime comedy film. “Chain... ... Wikipedia
Chain reaction scientific concept. And also “Chain Reaction” is the name of several feature films: “Chain Reaction” is a 1962 USSR film. "Chain Reaction" is a 1963 French crime comedy film. "Chain Reaction" Australian film... ... Wikipedia
Chain Reaction (film, 1963) This term has other meanings, see Chain Reaction (definitions). Chain reaction Carambolages ... Wikipedia
Books
- Chain Reaction, Elkeles Simone. Age 18+ 3 features: - The New York Times bestseller, Amazon - From the author of the world bestsellers "Perfect Chemistry" and "The Law of Attraction" - For those who believe that love changes everything "Excellent…
It is a process in which one reaction carried out causes subsequent reactions of the same type.
During the fission of one uranium nucleus, the resulting neutrons can cause the fission of other uranium nuclei, and the number of neutrons increases like an avalanche.
The ratio of the number of neutrons produced in one fission event to the number of such neutrons in the previous fission event is called the neutron multiplication factor k.
When k is less than 1, the reaction decays, because the number of absorbed neutrons is greater than the number of newly formed ones.
When k is greater than 1, an explosion occurs almost instantly.
When k equals 1, a controlled stationary chain reaction occurs.
The chain reaction is accompanied by the release of a large amount of energy.
To carry out a chain reaction, it is impossible to use any nuclei that fission under the influence of neutrons.
The chemical element uranium, used as fuel for nuclear reactors, naturally consists of two isotopes: uranium-235 and uranium-238.
In nature, uranium-235 isotopes make up only 0.7% of the total uranium reserve, but they are the ones that are suitable for carrying out a chain reaction, because fission under the influence of slow neutrons.
Uranium-238 nuclei can fission only under the influence of high-energy neutrons (fast neutrons). Only 60% of the neutrons produced during the fission of the uranium-238 nucleus have this energy. Approximately only 1 in 5 neutrons produced causes nuclear fission.
Conditions for a chain reaction in uranium-235:
The minimum amount of fuel (critical mass) required to carry out a controlled chain reaction in a nuclear reactor
- the speed of neutrons should cause fission of uranium nuclei
- absence of impurities that absorb neutrons
Critical mass:
If the mass of uranium is small, neutrons will fly outside of it without reacting
- if the mass of uranium is large, an explosion is possible due to a strong increase in the number of neutrons
- if the mass corresponds to the critical mass, a controlled chain reaction occurs
For uranium-235, the critical mass is 50 kg (this is, for example, a ball of uranium with a diameter of 9 cm).
The first controlled chain reaction - USA in 1942 (E. Fermi)
In the USSR - 1946 (I.V. Kurchatov).
Faraday's law of electromagnetic induction is the basic law of electrodynamics concerning the principles of operation of transformers, chokes, and many types of electric motors
And generators. The law states:
Faraday's law as two different phenomena[edit | edit wiki text]
Some physicists note that Faraday's law describes two different phenomena in one equation: motor EMF, generated by the action of a magnetic force on a moving wire, and transformer EMF, generated by the action of electric force due to changes in the magnetic field. James Clerk Maxwell drew attention to this fact in his work About physical lines of force in 1861. In the second half of Part II of this work, Maxwell gives a separate physical explanation for each of these two phenomena. Reference to these two aspects of electromagnetic induction is available in some modern textbooks. As Richard Feynman writes:
Lorentz's law[edit | edit wiki text]
Charge q in the conductor on the left side of the loop experiences the Lorentz force q v× B k = −q v B(x C − w / 2) j (j,k- unit vectors in directions y And z; see vector product of vectors), which causes emf (work per unit charge) v ℓ B(x C − w / 2) along the entire length of the left side of the loop. On the right side of the loop, a similar reasoning shows that the emf is equal to v ℓ B(x C + w / 2). Two emfs opposite each other push the positive charge towards the bottom of the loop. In case the field B increases along x, the force on the right side will be greater and the current will flow clockwise. Using the right hand rule, we find that the field B, created by the current, is opposite to the applied field. The emf causing the current must increase in a counterclockwise direction (as opposed to the current). Adding the emf in a counterclockwise direction along the loop we find:
Faraday's Law[edit | edit wiki text]
An intuitively attractive but flawed approach to using the flow rule expresses the flow through a circuit as Φ B = Bwℓ, where w- width of the moving loop. This expression is independent of time, so it incorrectly follows that no emf is generated. The error in this statement is that it does not take into account the entire path of the current through the closed loop.
To use the flow rule correctly, we must consider the entire current path, which includes the path through the rims on the upper and lower rims. We can choose an arbitrary closed path through the rims and the rotating loop, and using the flow law, find the emf along this path. Any path that includes a segment adjacent to a rotating loop takes into account the relative motion of the parts of the chain.
As an example, consider a path passing in the upper part of the chain in the direction of rotation of the upper disk, and in the lower part of the chain - in the opposite direction with respect to the lower disk (shown by arrows in Fig. 4). In this case, if the rotating loop has deviated by an angle θ from the collector loop, then it can be considered as part of a cylinder with an area A = rℓθ. This area is perpendicular to the field B, and its contribution to the flow is equal to:
where the sign is negative because according to the right-hand rule the field B , generated by a loop with current, opposite in direction to the applied field B". Since this is only the time-dependent part of the flux, according to the flux law the emf is:
![]()
in accordance with the formula of Lorentz's law.
Now consider another path, in which we choose to pass along the rims of the disks through opposite segments. In this case the associated thread will be decrease with increasing θ, but according to the right-hand rule, the current loop adds attached field B, therefore the EMF for this path will be exactly the same value as for the first path. Any mixed return path produces the same result for the emf value, so it doesn't really matter which path you take.
A thermonuclear reaction is a type of nuclear reaction in which light atomic nuclei combine into heavier ones due to the kinetic energy of their thermal motion. Origin of the term[edit | edit wiki text]
In order for a nuclear reaction to occur, the original atomic nuclei must overcome the so-called “Coulomb barrier” - the force of electrostatic repulsion between them. To do this, they must have high kinetic energy. According to kinetic theory, the kinetic energy of moving microparticles of a substance (atoms, molecules or ions) can be represented as temperature, and therefore, by heating the substance, a nuclear reaction can be achieved. It is this relationship between heating a substance and a nuclear reaction that is reflected by the term “thermonuclear reaction.”
Coulomb barrier[edit | edit wiki text]
Atomic nuclei have a positive electrical charge. At large distances, their charges can be shielded by electrons. However, in order for the fusion of nuclei to occur, they must approach each other to a distance at which the strong interaction operates. This distance is on the order of the size of the nuclei themselves and many times smaller than the size of an atom. At such distances, the electron shells of atoms (even if they were preserved) can no longer shield the charges of the nuclei, so they experience strong electrostatic repulsion. The force of this repulsion, in accordance with Coulomb's law, is inversely proportional to the square of the distance between the charges. At distances on the order of the size of the nuclei, the magnitude of the strong interaction, which tends to bind them, begins to increase rapidly and becomes greater than the magnitude of the Coulomb repulsion.
Thus, in order to react, nuclei must overcome a potential barrier. For example, for the deuterium-tritium reaction, the value of this barrier is approximately 0.1 MeV. For comparison, the ionization energy of hydrogen is 13 eV. Therefore, the substance participating in the thermonuclear reaction will be an almost completely ionized plasma.
The temperature equivalent to 0.1 MeV is approximately 10 9 K, however there are two effects that lower the temperature required for a fusion reaction:
· Firstly, temperature characterizes only the average kinetic energy; there are particles with both lower and higher energy. In fact, a thermonuclear reaction involves a small number of nuclei that have an energy much higher than the average (the so-called “tail of the Maxwellian distribution”
· Secondly, due to quantum effects, nuclei do not necessarily have an energy exceeding the Coulomb barrier. If their energy is slightly less than the barrier, they are more likely to tunnel through it. [ source not specified 339 days]
Thermonuclear reactions[edit | edit wiki text]
Some of the most important exothermic thermonuclear reactions with large cross sections:
| (1) | D | + | T | → | 4He | (3.5 MeV) | + | n | (14.1 MeV) | |||||||
| (2) | D | + | D | → | T | (1.01 MeV) | + | p | (3.02 MeV) | (50 %) | ||||||
| (3) | → | 3He | (0.82 MeV) | + | n | (2.45 MeV) | (50 %) | |||||||||
| (4) | D | + | 3He | → | 4He | (3.6 MeV) | + | p | (14.7 MeV) | |||||||
| (5) | T | + | T | → | 4He | + | n | + 11.3 MeV | ||||||||
| (6) | 3He | + | 3He | → | 4He | + | p | |||||||||
| (7) | 3He | + | T | → | 4He | + | p | + | n | + 12.1 MeV | (51 %) | |||||
| (8) | → | 4He | (4.8 MeV) | + | D | (9.5 MeV) | (43 %) | |||||||||
| (9) | → | 4He | (0.5 MeV) | + | n | (1.9 MeV) | + | p | (11.9 MeV) | (6 %) | ||||||
| (10) | D | + | 6Li | → | 4He | + 22.4 MeV - | ||||||||||
| (11) | p | + | 6Li | → | 4He | (1.7 MeV) | + | 3He | (2.3 MeV)- | |||||||
| (12) | 3He | + | 6Li | → | 4He | + | p | + 16.9 MeV | ||||||||
| (13) | p | + | 11B | → | 4He | + 8.7 MeV | ||||||||||
| (14) | n | + | 6Li | → | 4He | + | T | + 4.8 MeV |
Muon catalysis[edit | edit wiki text]
Main article: Muon catalysis
The thermonuclear reaction can be significantly facilitated by introducing negatively charged muons into the reaction plasma.
Muons µ − , interacting with thermonuclear fuel, form mesomolecules in which the distance between the nuclei of fuel atoms is somewhat smaller, which facilitates their approach and, in addition, increases the probability of tunneling of nuclei through the Coulomb barrier.
Number of synthesis reactions X c, initiated by one muon, is limited by the value of the muon sticking coefficient. Experimentally, it was possible to obtain values of X c ~ 100, i.e., one muon is capable of releasing energy ~ 100 × X MeV, where X is the energy output of the catalyzed reaction.
So far, the amount of released energy is less than the energy costs for the production of the muon itself (5-10 GeV). Thus, muon catalysis is still an energetically unfavorable process. Commercially viable energy production using muon catalysis is possible with X c ~ 10 4 .
Application[edit | edit wiki text]
The use of thermonuclear reaction as a practically inexhaustible source of energy is associated primarily with the prospect of mastering the technology of controlled thermonuclear fusion (CTF). Currently, the scientific and technological base does not allow the use of CTS on an industrial scale.
At the same time, uncontrolled thermonuclear reaction has found its application in military affairs. The first thermonuclear explosive device was tested in November 1952 in the United States, and already in August 1953, a thermonuclear explosive device in the form of an aerial bomb was tested in the Soviet Union. The power of a thermonuclear explosive device (unlike an atomic one) is limited only by the amount of material used to create it, which makes it possible to create explosive devices of almost any power.
TICKET 27 question 1
Self-induction phenomenon
We have already studied that a magnetic field arises near a conductor carrying current. We also studied that an alternating magnetic field generates a current (the phenomenon of electromagnetic induction). Let's consider an electrical circuit. When the current strength changes in this circuit, the magnetic field will change, as a result of which an additional induced current. This phenomenon is called self-induction, and the current arising in this case is called self-induction current.
The phenomenon of self-induction is the occurrence of an EMF in a conducting circuit, created as a result of a change in current strength in the circuit itself.


The inductance of a circuit depends on its shape and size, on the magnetic properties of the environment and does not depend on the current strength in the circuit.
The self-induction emf is determined by the formula:


The phenomenon of self-induction is similar to the phenomenon of inertia. Just as in mechanics it is impossible to instantly stop a moving body, so a current cannot instantly acquire a certain value due to the phenomenon of self-induction. If a coil is connected in series with the second lamp in a circuit consisting of two identical lamps connected in parallel to a current source, then when the circuit is closed, the first lamp lights up almost immediately, and the second with a noticeable delay.

When the circuit is opened, the current strength quickly decreases, and the resulting self-inductive emf prevents the decrease in the magnetic flux. In this case, the induced current is directed in the same way as the original one. The self-induced emf can be many times greater than the external emf. Therefore, light bulbs very often burn out when the lights are turned off.
Magnetic field energy
Magnetic field energy of a current-carrying circuit:

Radioactive radiation is the radiation that an isotope releases during decay. It has three varieties: alpha rays (flow of helium atomic nuclei), beta rays (flow of electrons) and gamma rays (electromagnetic radiation). For humans, the most dangerous is gamma radiation.
The dose of absorbed radiation is equal to the ratio of the energy received by the body to the body mass. The absorption dose is designated by the letter D and is measured in grays.
In practice, the unit of measurement is also the roentgen (R), equal to 2.58 times 10 to the power of minus 4 coulomb, divided by kilogram.
Absorbed radiation can accumulate over time, and its dose increases the longer the irradiation lasts.
The dose rate is determined by the ratio of the dose of absorbed radiation to the irradiation time. It is designated by the letter N and is measured in grays divided per second.
For humans, the lethal dose of absorbed radiation is equivalent to 6 Gy. The maximum permissible dose of radiation for humans is 0.05 Gy per year.
TICKET 28 Question 1
An elementary particle is a collective term that refers to micro-objects on a subnuclear scale that cannot be split into their component parts.
It should be borne in mind that some elementary particles ( electron, neutrino, quarks etc.) are currently considered unstructured and considered as primary fundamental particles . Other elementary particles (so-called composite particles, including the particles that make up the nucleus atom - protons And neutrons) have a complex internal structure, but, nevertheless, according to modern ideas, it is impossible to divide them into parts due to the effect confinement.
In total with antiparticles More than 350 elementary particles have been discovered. Of these, the photon, electron and muon neutrino, electron, proton and their antiparticles are stable. The remaining elementary particles decay spontaneously in a time from approximately 1000 seconds (for a free neutron) to a negligible fraction of a second (from 10 −24 to 10 −22, for resonances).
With electromagnetic oscillations, periodic changes in electric charge, current and voltage occur. Electromagnetic oscillations are divided into free, fading, forced and self-oscillations.
Free oscillations are called oscillations that occur in a system (capacitor and coil) after it is removed from an equilibrium position (when a charge is imparted to the capacitor). More precisely, free electromagnetic oscillations occur when a capacitor is discharged through an inductor. Forced oscillations are called oscillations in a circuit under the influence of an external periodically changing electromotive force.
The simplest system in which free electromagnetic oscillations are observed is oscillatory circuit. It consists of an inductor and a capacitor. This process will be repeated again and again. will arise electromagnetic vibrations due to the conversion of energy from the electric field of the capacitor. 
· The capacitor, charging from the battery, will initially acquire a maximum charge. His energy W e will be maximum (Fig. a).
· If the capacitor is shorted to a coil, then at this moment in time it will begin to discharge (Fig. b). Current will appear in the circuit. As the capacitor discharges, the current in the circuit and in the coil increases. Due to the phenomenon of self-induction, this does not happen instantly. Coil Energy W m becomes maximum (Fig. c).
· The induction current flows in the same direction. Electrical charges are again accumulated on the capacitor. The capacitor is recharged, i.e. The capacitor plate, previously positively charged, will become negatively charged. The energy of the capacitor becomes maximum. The current in this direction will stop, and the process will repeat in the opposite direction (Fig. d).  This process will be repeated over and over again. will arise electromagnetic vibrations due to the conversion of the energy of the electric field of the capacitor into the energy of the magnetic field of the current coil, and vice versa. If there are no losses (resistance R = 0), then the current strength, charge and voltage change over time according to a harmonic law. Oscillations that occur according to the law of cosine or sine are called harmonic. Equation of harmonic charge oscillation:
This process will be repeated over and over again. will arise electromagnetic vibrations due to the conversion of the energy of the electric field of the capacitor into the energy of the magnetic field of the current coil, and vice versa. If there are no losses (resistance R = 0), then the current strength, charge and voltage change over time according to a harmonic law. Oscillations that occur according to the law of cosine or sine are called harmonic. Equation of harmonic charge oscillation: ![]() .
.
A circuit in which there is no energy loss is an ideal oscillatory circuit. Period of electromagnetic oscillations in an ideal oscillatory circuit depends on the inductance of the coil and the capacitance of the capacitor and is found according to Thomson's formula where L is the inductance of the coil, C is the capacitance of the capacitor, T is the period of electric oscillations.
In a real oscillatory circuit, free electromagnetic oscillations will be fading  due to energy loss when heating the wires. For practical application, it is important to obtain undamped electromagnetic oscillations, and for this it is necessary to replenish the oscillatory circuit with electricity in order to compensate for energy losses from the undamped oscillation generator, which is an example of a self-oscillating system.
due to energy loss when heating the wires. For practical application, it is important to obtain undamped electromagnetic oscillations, and for this it is necessary to replenish the oscillatory circuit with electricity in order to compensate for energy losses from the undamped oscillation generator, which is an example of a self-oscillating system.
Ticket 29 question 1
Antiparticle - a twin particle of some other elementary particle, having the same mass and the same spin, differing from it in the signs of all other interaction characteristics (charges such as electric And color charges, baryon and lepton quantum numbers).
The very definition of what to call a “particle” in a particle-antiparticle pair is largely arbitrary. However, for a given choice of “particle,” its antiparticle is determined uniquely. The conservation of the baryon number in weak interaction processes makes it possible to determine the “particle” in any baryon-antibaryon pair from the chain of baryon decays. The choice of an electron as a “particle” in the electron-positron pair fixes (due to the conservation of the lepton number in processes weak interaction) determination of the state of a “particle” in a pair of electron neutrino-antineutrino. Transitions between leptons of different generations (type ) have not been observed, so the definition of a “particle” in each generation of leptons, generally speaking, can be made independently. Usually, by analogy with an electron, “particles” are called negatively charged leptons, which, while preserving the lepton number, determines the corresponding neutrino And antineutrino. For bosons the concept of “particle” can be fixed by definition, for example, hypercharge.
Nuclear chain reaction- a sequence of single nuclear reactions, each of which is caused by a particle that appeared as a reaction product at the previous step of the sequence. An example of a nuclear chain reaction is the fission chain reaction of the nuclei of heavy elements, in which the majority of fission events are initiated by neutrons produced by fission of nuclei in the previous generation.
Encyclopedic YouTube
1 / 3
Nuclear physics. Nuclear reactions. Nuclear fission chain reaction. nuclear power plant
Nuclear forces Binding energy of particles in the nucleus Fission of uranium nuclei Chain reaction
Nuclear reactions
Subtitles
Energy release mechanism
The transformation of a substance is accompanied by the release of free energy only if the substance has a reserve of energy. The latter means that microparticles of a substance are in a state with a rest energy greater than in another possible state to which a transition exists. A spontaneous transition is always prevented by an energy barrier, to overcome which the microparticle must receive a certain amount of energy from the outside - excitation energy. The exoenergetic reaction consists in the fact that in the transformation following excitation, more energy is released than is required to excite the process. There are two ways to overcome the energy barrier: either due to the kinetic energy of colliding particles, or due to the binding energy of the joining particle.
If we keep in mind the macroscopic scale of energy release, then all or initially at least some fraction of particles of the substance must have the kinetic energy necessary to excite reactions. This is achievable only by increasing the temperature of the medium to a value at which the energy of thermal motion approaches the energy threshold limiting the course of the process. In the case of molecular transformations, that is, chemical reactions, such an increase is usually hundreds of kelvins, but in the case of nuclear reactions it is at least 10 7 K due to the very high height of the Coulomb barriers of colliding nuclei. Thermal excitation of nuclear reactions is carried out in practice only during the synthesis of the lightest nuclei, in which the Coulomb barriers are minimal (thermonuclear fusion).
Excitation by joining particles does not require large kinetic energy, and, therefore, does not depend on the temperature of the medium, since it occurs due to unused bonds inherent in the attractive forces of particles. But to excite reactions, the particles themselves are necessary. And if we again mean not a separate act of reaction, but the production of energy on a macroscopic scale, then this is possible only when a chain reaction occurs. The latter occurs when the particles that excite the reaction reappear as products of an exoenergetic reaction.
Chain reactions
Chain reactions are widespread among chemical reactions, where the role of particles with unused bonds is played by free atoms or radicals. The chain reaction mechanism during nuclear transformations can be provided by neutrons that do not have a Coulomb barrier and excite nuclei upon absorption. The appearance of the necessary particle in the environment causes a chain of reactions that follow one after another, which continues until the chain breaks due to the loss of the reaction carrier particle. There are two main reasons for losses: the absorption of a particle without the emission of a secondary one and the departure of the particle beyond the volume of the substance supporting the chain process. If in each act of reaction only one carrier particle appears, then the chain reaction is called unbranched. An unbranched chain reaction cannot lead to energy release on a large scale.
If in each act of reaction or in some links of the chain more than one particle appears, then a branched chain reaction occurs, because one of the secondary particles continues the started chain, while the others give rise to new chains that branch again. True, processes that lead to chain breaks compete with the branching process, and the resulting situation gives rise to limiting or critical phenomena specific to branched chain reactions. If the number of broken circuits is greater than the number of new circuits appearing, then self-sustaining chain reaction(SCR) turns out to be impossible. Even if it is excited artificially by introducing a certain amount of necessary particles into the medium, then, since the number of chains in this case can only decrease, the process that has begun quickly fades out. If the number of new chains formed exceeds the number of breaks, the chain reaction quickly spreads throughout the entire volume of the substance when at least one initial particle appears.
The region of states of matter with the development of a self-sustaining chain reaction is separated from the region where a chain reaction is generally impossible, critical condition. The critical state is characterized by equality between the number of new circuits and the number of breaks.
Achieving a critical state is determined by a number of factors. The fission of a heavy nucleus is excited by one neutron, and as a result of the fission act more than one neutron appears (for example, for 235 U the number of neutrons produced in one fission act is on average from 2 to 3). Consequently, the fission process can give rise to a branched chain reaction, the carriers of which will be neutrons. If the rate of neutron losses (captures without fission, escapes from the reaction volume, etc.) compensates for the rate of neutron multiplication in such a way that the effective neutron multiplication coefficient is exactly equal to unity, then the chain reaction proceeds in a stationary mode. The introduction of negative feedback between the effective multiplication factor and the rate of energy release allows for a controlled chain reaction, which is used, for example, in nuclear energy. If the multiplication factor is greater than one, the chain reaction develops exponentially; uncontrolled fission chain reaction is used in
Let's consider the mechanism of the fission chain reaction. When heavy nuclei fission under the influence of neutrons, new neutrons are produced. For example, with each fission of the uranium 92 U 235 nucleus, an average of 2.4 neutrons are produced. Some of these neutrons can again cause nuclear fission. This avalanche-like process is called chain reaction
.
The fission chain reaction occurs in an environment in which the process of neutron multiplication occurs. This environment is called core
. The most important physical quantity characterizing the intensity of neutron multiplication is neutron multiplication factor in the medium
k ∞ . The multiplication coefficient is equal to the ratio of the number of neutrons in one generation to their number in the previous generation. The index ∞ indicates that we are talking about an ideal environment of infinite dimensions. Similarly to the value k ∞ is determined neutron multiplication factor in a physical system
k. The k factor is a characteristic of a specific installation.
In a fissile medium of finite dimensions, some neutrons will escape from the core to the outside. Therefore, the coefficient k also depends on the probability P for a neutron not to escape from the core. By definition
| k = k ∞ P. | (1) |
The value of P depends on the composition of the active zone, its size, shape, and also on the extent to which the substance surrounding the active zone reflects neutrons.
The important concepts of critical mass and critical dimensions are associated with the possibility of neutrons leaving the core. Critical size
is the size of the active zone at which k = 1. Critical mass
is called the mass of the core of critical dimensions. It is obvious that at a mass below the critical one, the chain reaction does not occur, even if > 1. On the contrary, a noticeable excess of the mass above the critical one leads to an uncontrolled reaction - an explosion.
If in the first generation there are N neutrons, then in the nth generation there will be Nk n. Therefore, at k = 1 the chain reaction proceeds stationary, at k< 1 реакция гаснет, а при k >1 the intensity of the reaction increases. When k = 1 the reaction mode is called critical
, for k > 1 – supercritical
and at k< 1 – subcritical
.
The lifetime of one generation of neutrons strongly depends on the properties of the medium and is on the order of 10–4 to 10–8 s. Due to the smallness of this time, in order to carry out a controlled chain reaction, it is necessary to maintain the equality k = 1 with great accuracy, since, say, at k = 1.01 the system will explode almost instantly. Let's see what factors determine the coefficients k ∞ and k.
The first quantity that determines k ∞ (or k) is the average number of neutrons emitted in one fission event. The number depends on the type of fuel and the energy of the incident neutron. In table Table 1 shows the values of the main isotopes of nuclear energy for both thermal and fast (E = 1 MeV) neutrons.
The energy spectrum of fission neutrons for the 235 U isotope is shown in Fig. 1. Spectra of this kind are similar for all fissile isotopes: there is a strong scatter in energies, with the bulk of neutrons having energies in the range of 1–3 MeV. The neutrons produced during fission slow down, diffuse over a certain distance and are absorbed either with or without fission. Depending on the properties of the medium, neutrons have time to slow down to different energies before absorption. In the presence of a good moderator, the majority of neutrons have time to slow down to thermal energies of the order of 0.025 eV. In this case the chain reaction is called slow, or, what is the same, thermal. In the absence of a special moderator, neutrons only have time to slow down to energies of 0.1–0.4 MeV, since all fissile isotopes are heavy and therefore slow down poorly. The corresponding chain reactions are called fast(we emphasize that the epithets “fast” and “slow” characterize the speed of neutrons, and not the speed of the reaction). Chain reactions in which neutrons are slowed down to energies ranging from tens to one keV are called intermediate
.
When a neutron collides with a heavy nucleus, radiative capture of a neutron (n, γ) is always possible. This process will compete with division and thereby reduce the multiplication rate. It follows from this that the second physical quantity that affects the coefficients k ∞ , k is the probability of fission when a neutron is captured by the nucleus of a fissile isotope. This probability for monoenergetic neutrons is obviously equal to
| , | (2) |
where nf, nγ are the fission and radiation capture cross sections, respectively. To simultaneously take into account both the number of neutrons per fission event and the probability of radiative capture, a coefficient η is introduced, equal to the average number of secondary neutrons per neutron capture by a fissile nucleus.
| , | (3) |
the value of η depends on the type of fuel and on the neutron energy. The values of η for the most important isotopes for thermal and fast neutrons are given in the same table. 1. The value of η is the most important characteristic of fuel nuclei. A chain reaction can only occur when η > 1. The higher the value of η, the higher the quality of the fuel.
Table 1. Values of ν, η for fissile isotopes
| Core | 92 U 233 | 92 U 235 | 94 Pu 239 | |
| Thermal neutrons (E = 0.025 eV) |
ν | 2.52 | 2.47 | 2.91 |
| η | 2.28 | 2.07 | 2.09 | |
| Fast neutrons (E = 1 MeV) |
ν | 2.7 | 2.65 | 3.0 |
| η | 2.45 | 2.3 | 2.7 | |
The quality of nuclear fuel is determined by its availability and coefficient η. Only three isotopes are found in nature that can serve as nuclear fuel or raw materials for its production. These are the isotope of thorium 232 Th and the isotopes of uranium 238 U and 235 U. Of these, the first two do not give a chain reaction, but can be processed into isotopes on which the reaction occurs. The 235 U isotope itself gives a chain reaction. There is several times more thorium in the earth's crust than uranium. Natural thorium practically consists of only one isotope, 232 Th. Natural uranium consists mainly of the 238 U isotope and only 0.7% of the 235 U isotope.
In practice, the question of the feasibility of a chain reaction on a natural mixture of uranium isotopes, in which there are 140 238 U nuclei per 235 U nucleus, is extremely important. Let us show that on a natural mixture a slow reaction is possible, but a fast one is not. To consider a chain reaction in a natural mixture, it is convenient to introduce a new quantity - the average neutron absorption cross section per one nucleus of the 235 U isotope. By definition
For thermal neutrons = 2.47, = 580 barn, = 112 barn, = 2.8 barn (note how small the last cross section is). Substituting these figures into (5), we obtain that for slow neutrons in a natural mixture
This means that 100 thermal neutrons, absorbed in the natural mixture, will create 132 new neutrons. It directly follows from this that a chain reaction with slow neutrons is in principle possible on natural uranium. In principle, because to actually implement a chain reaction, you need to be able to slow down neutrons with low losses.
For fast neutrons ν = 2.65, 2 barn, 0.1 barn. If we take into account fission only on the 235 U isotope, we obtain
| 235 (fast) 0.3. | (7) |
But we must also take into account that fast neutrons with energies greater than 1 MeV can, with noticeable relative intensity, divide the nuclei of the 238 U isotope, which is very abundant in the natural mixture. For division by 238 U, the coefficient is approximately 2.5. In the fission spectrum, approximately 60% of neutrons have energies above the effective threshold of 1.4 MeV fission by 238 U. But of these 60%, only one neutron out of 5 manages to fission without slowing down to an energy below the threshold due to elastic and especially inelastic scattering. From here, for the coefficient 238 (fast) we get the estimate
Thus, a chain reaction in a natural mixture (235 U + 238 U) cannot occur with fast neutrons. It has been experimentally established that for pure metallic uranium the multiplication factor reaches a value of unity with an enrichment of 5.56%. In practice, it turns out that the reaction with fast neutrons can only be maintained in an enriched mixture containing at least 15% of the 235 U isotope.
A natural mixture of uranium isotopes can be enriched with the 235 U isotope. Enrichment is a complex and expensive process due to the fact that the chemical properties of both isotopes are almost the same. It is necessary to take advantage of small differences in the rates of chemical reactions, diffusion, etc., arising due to differences in the masses of isotopes. The chain reaction with 235 U is almost always carried out in an environment with a high content of 238 U. A natural mixture of isotopes is often used, for which η = 1.32 in the thermal neutron region, since 238 U is also useful. The 238 U isotope is fissile by neutrons with energies above 1 MeV. This fission results in a small additional multiplication of neutrons.
Let's compare fission chain reactions with thermal and fast neutrons.
For thermal neutrons, the capture cross sections are large and vary greatly when passing from one nucleus to another. On the nuclei of some elements (for example, cadmium), these cross sections are hundreds or more times higher than the cross sections on 235 U. Therefore, high purity requirements are imposed on the core of thermal neutron installations in relation to certain impurities.
For fast neutrons, all capture cross sections are small and not so different from each other, so the problem of high purity of materials does not arise. Another advantage of fast reactions is a higher reproduction rate.
An important distinctive property of thermal reactions is that in the core the fuel is much more diluted, i.e., per fuel core there are significantly more nuclei not participating in fission than in a fast reaction. For example, in a thermal reaction on natural uranium, there are 140 nuclei of 238 U raw material per 235 U fuel core, and in a fast reaction, there can be no more than five to six 238 U nuclei per 235 U nucleus. The dilution of fuel in a thermal reaction leads to the fact that one and the same energy in a thermal reaction is released in a much larger volume of matter than in a rapid reaction. Thus, it is easier to remove heat from the active zone of a thermal reaction, which allows this reaction to be carried out with greater intensity than a fast one.
The lifetime of one generation of neutrons for a fast reaction is several orders of magnitude shorter than for a thermal one. Therefore, the rate of a fast reaction can change noticeably within a very short time after a change in the physical conditions in the core. During normal operation of the reactor, this effect is insignificant, since in this case the operating mode is determined by the lifetimes of delayed rather than prompt neutrons.
In a homogeneous medium consisting only of fissile isotopes of one type, the multiplication factor would be equal to η. However, in real situations, in addition to fissile nuclei, there are always other, non-fissionable ones. These extraneous nuclei will capture neutrons and thereby affect the multiplication factor. It follows that the third quantity determining the coefficients k ∞ , k, is the probability that the neutron will not be captured by one of the non-fissile nuclei. In real installations, “foreign” capture occurs on the moderator cores, on the cores of various structural elements, as well as on the cores of fission products and capture products.
To carry out a chain reaction with slow neutrons, special substances are introduced into the core - moderators, which convert fission neutrons into thermal ones. In practice, the slow neutron chain reaction is carried out on natural or slightly enriched uranium with the 235 U isotope. The presence of a large amount of the 238 U isotope in the core complicates the moderation process and makes it necessary to place high demands on the quality of the moderator. The life of one generation of neutrons in a core with a moderator can be approximately divided into two stages: moderation to thermal energies and diffusion. thermal rates before absorption. In order for the majority of neutrons to have time to slow down without absorption, the condition must be met
where σ control, σ capture are the energy-averaged cross sections for elastic scattering and capture, respectively, and n is the number of neutron collisions with moderator nuclei required to achieve thermal energy. The number n increases rapidly with increasing mass number of the moderator. For uranium 238 U, the number n is of the order of several thousand. And the ratio σ control /σ capture for this isotope, even in the relatively favorable energy region of fast neutrons, does not exceed 50. The so-called resonance region from 1 keV to 1 eV is especially “dangerous” in relation to neutron capture. In this region, the total cross section for the interaction of a neutron with 238 U nuclei has a large number of intense resonances (Fig. 2). At low energies, the radiation widths exceed the neutron widths. Therefore, in the resonance region, the ratio σ control/σ capture becomes even less than unity. This means that when a neutron enters the region of one of the resonances, it is absorbed with almost one hundred percent probability. And since the slowdown on such a heavier nucleus as uranium occurs in “small steps,” then when passing through the resonant region, the slowing down neutron will definitely “bump into” one of the resonances and be absorbed. It follows that a chain reaction cannot be carried out on natural uranium without foreign impurities: on fast neutrons the reaction does not occur due to the small coefficient η, and slow neutrons cannot be formed. In order to avoid resonant neutron capture, very light nuclei must be used to slow them down , in which the slowdown occurs in “large steps,” which sharply increases the probability of a neutron successfully “slipping” through the resonant energy region. The best moderating elements are hydrogen, deuterium, beryllium, and carbon. Therefore, moderators used in practice mainly come down to heavy water, beryllium, beryllium oxide, graphite, as well as ordinary water, which slows down neutrons no worse than heavy water, but absorbs them in much larger quantities. The retarder must be well cleaned. Note that to carry out a slow reaction, the moderator must be tens or even hundreds of times more than uranium in order to prevent resonant collisions of neutrons with 238 U nuclei.
The moderating properties of the active medium can be approximately described by three quantities: the probability of a neutron avoiding absorption by a moderator during moderation, the probability p of avoiding resonant capture by 238 U nuclei, and the probability f of a thermal neutron being absorbed by a fuel nucleus rather than a moderator. The value f is usually called the thermal utilization coefficient. Accurate calculation of these quantities is difficult. Usually, approximate semi-empirical formulas are used to calculate them.
The values of p and f depend not only on the relative amount of the moderator, but also on the geometry of its placement in the core. The active zone, consisting of a homogeneous mixture of uranium and moderator, is called homogeneous, and the system of their alternating blocks of uranium and moderator is called heterogeneous (Fig. 4). A qualitatively heterogeneous system is distinguished by the fact that in it the fast neutron formed in uranium manages to go into the moderator without reaching resonant energies. Further deceleration occurs in a pure moderator. This increases the probability p of avoiding resonant capture
p het > p homo.
On the other hand, on the contrary, having become thermal in the moderator, the neutron must, in order to participate in the chain reaction, diffuse, without being absorbed in the pure moderator, to its boundary. Therefore, the thermal utilization factor f in a heterogeneous environment is lower than in a homogeneous one:
f get< f гом.
To estimate the multiplication factor k ∞ of a thermal reactor, an approximate four factor formula
| k∞ = η pfε . | (11) |
We have already considered the first three factors earlier. The quantity ε is called fast neutron multiplication factor
. This coefficient is introduced in order to take into account that some fast neutrons can fission without having time to slow down. In its meaning, the coefficient ε always exceeds one. But this excess is usually small. Typical for thermal reactions is the value ε = 1.03. For fast reactions, the formula of four factors is not applicable, since each coefficient depends on energy and the energy spread in fast reactions is very large.
Since the value of η is determined by the type of fuel, and the value of ε for slow reactions almost does not differ from unity, the quality of a particular active medium is determined by the product pf. Thus, the advantage of a heterogeneous medium over a homogeneous medium is quantitatively manifested in the fact that, for example, in a system in which there are 215 graphite nuclei per natural uranium nucleus, the product pf is equal to 0.823 for a heterogeneous medium and 0.595 for a homogeneous one. And since for a natural mixture η = 1.34, we get that for a heterogeneous medium k ∞ > 1, and for a homogeneous medium k ∞< 1.
For the practical implementation of a stationary chain reaction, one must be able to control this reaction. This control is greatly simplified due to the emission of delayed neutrons during fission. The overwhelming majority of neutrons escape from the nucleus almost instantly (i.e., in a time that is many orders of magnitude shorter than the lifetime of a generation of neutrons in the core), but several tenths of a percent of neutrons are delayed and escape from fragment nuclei after a fairly large period of time - from fractions seconds to several and even tens of seconds. The effect of delayed neutrons can be qualitatively explained as follows. Let the multiplication factor instantly increase from a subcritical value to such a supercritical value that k< 1 при отсутствии
запаздывающих нейтронов. Тогда, очевидно, цепная реакция начнется не сразу, а
лишь после вылета запаздывающих нейтронов. Тем самым процесс течения реакции
будет регулируемым, если время срабатывания регулирующих устройств будет меньше
сравнительно большого времени задержки запаздывающих нейтронов, а не очень
малого времени развития цепной реакции. Доля запаздывающих нейтронов в ядерных
горючих колеблется от 0.2 до 0.7%. Среднее время жизни запаздывающих нейтронов
составляет приблизительно 10 с. При небольшой степени надкритичности скорость
нарастания интенсивности цепной реакции определяется только запаздывающими
нейтронами.
The capture of neutrons by nuclei not participating in the chain reaction reduces the intensity of the reaction, but can be beneficial in relation to the formation of new fissile isotopes. Thus, when neutrons are absorbed from the isotopes of uranium 238 U and thorium 232 Th, the isotopes of plutonium 239 Pu and uranium 233 U are formed (through two successive β-decays), which are nuclear fuel:
| , | (12) |
| . | (13) |
These two reactions open up a real possibility reproduction of nuclear fuel during a chain reaction. In the ideal case, i.e., in the absence of unnecessary losses of neutrons, an average of 1 neutron can be used for reproduction for each act of absorption of a neutron by a fuel nucleus.
Nuclear (atomic) reactors
A reactor is a device in which a controlled fission chain reaction is maintained. When the reactor operates, heat is released due to the exothermic nature of the fission reaction. The main characteristic of a reactor is its power - the amount of thermal energy released per unit time. The reactor power is measured in megawatts (10 6 W). A power of 1 MW corresponds to a chain reaction in which 3·1016 fission events occur per second. There are a large number of different types of reactors. One of the typical schemes of a thermal reactor is shown in Fig. 5.
The main part of the reactor is the active zone in which the reaction occurs and thereby releases energy. In thermal and intermediate neutron reactors, the core consists of a fuel, usually mixed with a non-fissile isotope (usually 238 U), and a moderator. There is no moderator in the core of fast neutron reactors.
The core volume varies from tenths of a liter in some fast neutron reactors to tens of cubic meters in large thermal reactors. To reduce neutron leakage, the core is given a spherical or nearly spherical shape (for example, a cylinder with a height approximately equal to the diameter, or a cube).
Depending on the relative location of the fuel and moderator, homogeneous and heterogeneous reactors are distinguished. An example of a homogeneous active zone is a solution of uranyl sulfate salt and U 2 SO 4 in ordinary or heavy water. Heterogeneous reactors are more common. In heterogeneous reactors, the core consists of a moderator in which cassettes containing fuel are placed. Since energy is released in these cassettes, they are called fuel elements
or for short fuel rods. The active zone with reflector is often enclosed in a steel casing.
- The role of delayed neutrons in nuclear reactor control
A chain reaction is a self-sustaining chemical reaction in which initially appearing products take part in the formation of new products. Chain reactions usually occur at high speed and often have the character of an explosion.
Chain reactions go through three main stages: origin (initiation), development and chain termination.
Rice. 9.13. The energy profile of a reaction (a plot of potential energy versus reaction coordinate) showing a minimum that corresponds to the formation of a reaction intermediate.
Initiation stage. At this stage, the formation of intermediates (intermediate products) occurs. Intermediates can be atoms, ions or neutral molecules. Initiation can be accomplished by light, nuclear radiation, thermal (thermal) energy, anions, or catalysts.
Stage of development. At this stage, intermediates react with the original reactants to form new intermediates and final products. The development stage in chain reactions is repeated many times, which leads to the formation of a large number of final and intermediate products.
Circuit break stage. At this stage, the final consumption of intermediate products or their destruction occurs. As a result, the reaction stops. The chain reaction can break spontaneously or under the influence of special substances - inhibitors.
Chain reactions play an important role in many branches of chemistry, in particular in photochemistry, combustion chemistry, nuclear fission and nuclear fusion reactions (see Chapter 1), and organic chemistry (see Chapters 17-20).
Photochemistry
This branch of chemistry covers chemical processes associated with the effect of light on matter. An example of a photochemical process is photosynthesis.
Many chain reactions are initiated by light. The initiating particle in this case is a photon, which has energy (see Section 1.2). A classic example is the reaction between hydrogen and chlorine in the presence of light
This reaction proceeds explosively. It includes the following three stages.
Initiation. At this stage, the covalent bond in the chlorine molecule is broken, resulting in the formation of two atoms, each with an unpaired electron:
A reaction of this type is homolysis, or hemolytic division (see Section 17.3). It is also an example of photolysis. The term photolysis means photochemical decomposition. The two chlorine atoms formed are intermediates. They are radicals. A radical is an atom (or group of atoms) that has at least one unpaired electron. It should be noted that although the initiation stage is the slowest stage of the chain reaction, it does not determine the speed of the entire chain reaction.
Stage of development. At this stage, chlorine atoms react with hydrogen molecules, forming the final product - hydrogen chloride, as well as hydrogen radicals. Hydrogen radicals react with chlorine molecules; as a result, new portions of the product and new chlorine radicals are formed:
![]()
These two reactions, which together make up the developmental stage, are repeated millions of times.
Circuit break stage. The chain reaction finally stops as a result
reactions such as

To absorb the energy that is released during these chain termination reactions, it is necessary for some third body to take part in them. This third body is usually the walls of the vessel in which the reaction is carried out.
Quantum yield
The absorption of one photon of light by a chlorine molecule in the chain reaction described above can result in the formation of millions of hydrogen chloride molecules. The ratio of the number of product molecules to the number of light quanta (photons) initiating the reaction is called the quantum yield. The quantum yield of photochemical reactions can range from one to several millions. A high quantum yield indicates the chain nature of the reaction occurring.
Pulse photolysis
This is the name of the technique used to obtain radicals at a concentration high enough to detect them. In Fig. Figure 9.14 shows a simplified diagram of the setup used for flash photolysis. The reaction mixture is affected

Rice. 9.14. Pulsed photolysis.
with a powerful flash of light from a special pulsed source. Such a source makes it possible to create flashes of light with an energy of up to 105 J and with a duration of the order of s or less. Modern methods of pulsed photolysis use pulsed lasers with a flash duration of the order of a nanosecond (10-9 s). The reaction occurring as a result of such a flash of light can be monitored by recording a sequence of optical absorption spectra of the reaction mixture. The first flash is followed by a series of flashes from a low-power pulsed source. These flashes follow each other at intervals of the order of milliseconds or microseconds and make it possible to record the absorption spectra of the reaction mixture at such time intervals.
Combustion
The reaction with oxygen, resulting in the release of heat energy and light, is called combustion. Combustion usually occurs as a complex sequence of radical reactions.
Let's take hydrogen combustion as an example. Under certain conditions, this reaction occurs explosively. In Fig. Figure 9.15 presents experimental data for the reaction of a stoichiometric mixture of hydrogen and oxygen in a Pyrex reactor. The shaded area of the diagram corresponds to the explosive region of this reaction. For the hydrogen combustion reaction, this section of the diagram has the shape of an explosive peninsula. The explosion area is limited by the boundaries of the explosion.

Rice. 9.15. Conditions for the explosive occurrence of the hydrogen combustion reaction: