Tungsten is the most refractory of metals. Only the non-metallic element carbon has a higher melting point, but it exists in liquid form only at high pressures. Under standard conditions, tungsten is chemically resistant.
History and origin of the name
The name Wolframium was transferred to the element from the mineral wolframite, known back in the 16th century. called “wolf foam” - lat. spuma lupi or German. Wolf Rahm. The name was due to the fact that tungsten, accompanying tin ores, interfered with the smelting of tin, turning it into slag foam (“it devoured tin like a wolf devouring a sheep”).
Physical properties
Tungsten is a shiny light gray metal that has the highest proven melting and boiling points (it is assumed that seaborgium is even more refractory, but so far this cannot be firmly stated - the lifetime of seaborgium is very short). Melting point - 3695 (3422 °C), boils at 5828 (5555 °C). The density of pure tungsten is 19.25 g/cm³. It has paramagnetic properties (magnetic susceptibility 0.32⋅10 −9). Brinell hardness 488 kg/mm², electrical resistivity at 20 °C - 55⋅10 −9 Ohm m, at 2700 °C - 904⋅10 −9 Ohm m. The speed of sound in annealed tungsten is 4290 m/s.
Tungsten is one of the heaviest, hardest and most refractory metals. In its pure form, it is a silver-white metal, similar to platinum, at a temperature of about 1600 ° C it is easily forged and can be drawn into a thin thread. The metal is highly stable in a vacuum.
Chemical properties
2 W + 4 H N O 3 + 10 H F ⟶ W F 6 + W O F 4 + 4 N O + 7 H 2 O (\displaystyle (\mathsf (2W+4HNO_(3)+10HF\longrightarrow WF_(6)+WOF_(4)+ 4NO\uparrow +7H_(2)O)))Reacts with molten alkalis in the presence of oxidizing agents:
2 W + 4 N a O H + 3 O 2 ⟶ 2 N a 2 W O 4 + 2 H 2 O (\displaystyle (\mathsf (2W+4NaOH+3O_(2)\longrightarrow 2Na_(2)WO_(4)+2H_ (2)O))) W + 2 N a O H + 3 N a N O 3 ⟶ N a 2 W O 4 + 3 N a N O 2 + H 2 O (\displaystyle (\mathsf (W+2NaOH+3NaNO_(3)\longrightarrow Na_(2)WO_ (4)+3NaNO_(2)+H_(2)O)))At first, these reactions proceed slowly, but when they reach 400 °C (500 °C for a reaction involving oxygen), tungsten begins to self-heat, and the reaction proceeds quite violently, producing a large amount of heat.
It dissolves in a mixture of nitric and hydrofluoric acid, forming hexafluorotungstic acid H2. Of the tungsten compounds, the most important are: tungsten trioxide or tungsten anhydride, tungstates, peroxide compounds with the general formula Me 2 WO X, as well as compounds with halogens, sulfur and carbon. Tungstates are prone to forming polymer anions, including heteropolycompounds with the inclusion of other transition metals.
Application
The main use of tungsten is as the basis of refractory materials in metallurgy.
Tungsten metal
Tungsten connections
- For mechanical processing of metals and non-metallic structural materials in mechanical engineering (turning, milling, planing, chiselling), well drilling, and in the mining industry, hard alloys and composite materials based on tungsten carbide are widely used (for example, win, consisting of WC crystals in a cobalt matrix; grades widely used in Russia - VK2, VK4, VK6, VK8, VK15, VK25, T5K10, T15K6, T30K4), as well as mixtures of tungsten carbide, titanium carbide, tantalum carbide (TT grades for particularly difficult processing conditions, for example, chiselling and planing forgings made of heat-resistant steels and rotary hammer drilling of strong materials). Widely used as an alloying element (often together with molybdenum) in steels and iron-based alloys. High-alloy steel, classified as “high-speed”, with a marking starting with the letter P, almost always contains tungsten.
- Tungsten sulfide WS 2 is used as a high-temperature (up to 500 °C) lubricant.
- Some tungsten compounds are used as catalysts and pigments.
- Single crystals of tungstates (lead, cadmium, calcium tungstates) are used as scintillation detectors of X-rays and other ionizing radiation in nuclear physics and nuclear medicine.
- Tungsten ditelluride WTe 2 is used to convert thermal energy into electrical energy (thermo-emf about 57 μV/K).
Other Applications
Tungsten Market
Prices for metal tungsten (element content of about 99%) at the end of 2010 were about 40-42 US dollars per kilogram, in May 2011 they were about 53-55 US dollars per kilogram. Semi-finished products from 58 USD (rods) to 168 (thin strip). In 2014, tungsten prices fluctuated in the range from 55 to 57 USD.
Biological role
Tungsten does not play a significant biological role. Some archaebacteria and bacteria have enzymes that include tungsten in their active center. There are obligate tungsten-dependent forms of hyperthermophilic archaebacteria that live around deep-sea hydrothermal vents. The presence of tungsten in enzymes can be considered a physiological relic of early Archaea - there are suggestions that tungsten played a role in the early stages of the origin of life.
Natural tungsten consists of a mixture of five isotopes (180 W - 0.12(1)%, 182 W - 26.50(16)%, 183 W - 14.31(4)%, 184 W - 30.64(2) % and 186 W - 28.43(19) %). The extremely weak radioactivity of natural tungsten was discovered (about two decays per gram of element per year), due to the α-activity of 180 W, which has a half-life of 1.8⋅10 18 years.
Notes
- Michael E. Wieser, Norman Holden, Tyler B. Coplen, John K. Böhlke, Michael Berglund, Willi A. Brand, Paul De Bièvre, Manfred Gröning, Robert D. Loss, Juris Meija, Takafumi Hirata, Thomas Prohaska, Ronny Schoenberg, Glenda O'Connor, Thomas Walczyk, Shige Yoneda, Xiang-Kun Zhu. Atomic weights of the elements 2011 (IUPAC Technical Report) // Pure and Applied Chemistry. - 2013. - Vol. 85, no. 5. - P. 1047-1078. - DOI:10.1351/PAC-REP-13-03-02.
- Tungsten: physical properties(English) . WebElements. Retrieved August 17, 2013.
Tungsten- the most refractory of metals. Only the non-metallic element, carbon, has a higher melting point. Under standard conditions it is chemically resistant. The name Wolframium was transferred to the element from the mineral wolframite, known back in the 16th century. called lat. Spuma lupi (“wolf foam”) or German. Wolf Rahm (“wolf cream”, “wolf cream”). The name was due to the fact that tungsten, accompanying tin ores, interfered with the smelting of tin, turning it into foam of slag (“tin devours like a wolf devouring a sheep”).
See also:
STRUCTURE
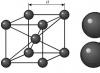
 Tungsten crystal has a body-centered cubic lattice. Tungsten crystals in the cold are characterized by low plasticity, therefore, during the process of pressing the powder, they practically do not change their basic shape and size, and the compaction of the powder occurs mainly through the relative movement of particles.
Tungsten crystal has a body-centered cubic lattice. Tungsten crystals in the cold are characterized by low plasticity, therefore, during the process of pressing the powder, they practically do not change their basic shape and size, and the compaction of the powder occurs mainly through the relative movement of particles. In a body-centered cubic tungsten cell, the atoms are located at the vertices and in the center of the cell, i.e. There are two atoms per cell. The bcc structure is not the closest packing of atoms. The compactness coefficient is 0.68. Tungsten space group Im3m.
PROPERTIES
 Tungsten is a shiny light gray metal that has the highest proven melting and boiling points (it is assumed that seaborgium is even more refractory, but so far this cannot be firmly stated - the lifetime of seaborgium is very short). Melting point - 3695 K (3422 °C), boils at 5828 K (5555 °C). The density of pure tungsten is 19.25 g/cm³. It has paramagnetic properties (magnetic susceptibility 0.32·10−9). Brinell hardness 488 kg/mm², electrical resistivity at 20 °C - 55·10−9 Ohm·m, at 2700 °C - 904·10−9 Ohm·m. The speed of sound in annealed tungsten is 4290 m/s. Is paramagnetic.
Tungsten is a shiny light gray metal that has the highest proven melting and boiling points (it is assumed that seaborgium is even more refractory, but so far this cannot be firmly stated - the lifetime of seaborgium is very short). Melting point - 3695 K (3422 °C), boils at 5828 K (5555 °C). The density of pure tungsten is 19.25 g/cm³. It has paramagnetic properties (magnetic susceptibility 0.32·10−9). Brinell hardness 488 kg/mm², electrical resistivity at 20 °C - 55·10−9 Ohm·m, at 2700 °C - 904·10−9 Ohm·m. The speed of sound in annealed tungsten is 4290 m/s. Is paramagnetic.
Tungsten is one of the heaviest, hardest and most refractory metals. In its pure form, it is a silver-white metal, similar to platinum, at a temperature of about 1600 ° C it is easily forged and can be drawn into a thin thread.
RESERVES AND PRODUCTION
 The tungsten Clarke of the earth's crust is (according to Vinogradov) 1.3 g/t (0.00013% of the content in the earth's crust). Its average content in rocks, g/t: ultrabasic - 0.1, basic - 0.7, intermediate - 1.2, acidic - 1.9.
The tungsten Clarke of the earth's crust is (according to Vinogradov) 1.3 g/t (0.00013% of the content in the earth's crust). Its average content in rocks, g/t: ultrabasic - 0.1, basic - 0.7, intermediate - 1.2, acidic - 1.9.
The process of obtaining tungsten goes through the substage of separation of trioxide WO 3 from ore concentrates and subsequent reduction to metal powder with hydrogen at a temperature of about 700 °C. Due to the high melting point of tungsten, powder metallurgy methods are used to obtain a compact shape: the resulting powder is pressed, sintered in a hydrogen atmosphere at a temperature of 1200-1300 °C, then an electric current is passed through it. The metal is heated to 3000 °C, and sintering occurs into a monolithic material. For subsequent purification and obtaining a single-crystalline form, zone melting is used.
ORIGIN
 Tungsten occurs in nature mainly in the form of oxidized complex compounds formed by tungsten trioxide WO 3 with oxides of iron and manganese or calcium, and sometimes lead, copper, thorium and rare earth elements. Wolframite (iron and manganese tungstate nFeWO 4 * mMnWO 4 - ferberite and hübnerite, respectively) and scheelite (calcium tungstate CaWO 4) are of industrial importance. Tungsten minerals are usually embedded in granite rocks, so the average tungsten concentration is 1-2%.
Tungsten occurs in nature mainly in the form of oxidized complex compounds formed by tungsten trioxide WO 3 with oxides of iron and manganese or calcium, and sometimes lead, copper, thorium and rare earth elements. Wolframite (iron and manganese tungstate nFeWO 4 * mMnWO 4 - ferberite and hübnerite, respectively) and scheelite (calcium tungstate CaWO 4) are of industrial importance. Tungsten minerals are usually embedded in granite rocks, so the average tungsten concentration is 1-2%.
Kazakhstan, China, Canada and the USA have the largest reserves; deposits are also known in Bolivia, Portugal, Russia, Uzbekistan and South Korea. World tungsten production is 49-50 thousand tons per year, including 41 in China, 3.5 in Russia; Kazakhstan 0.7, Austria 0.5. Main exporters of tungsten: China, South Korea, Austria. Main importers: USA, Japan, Germany, UK.
There are also tungsten deposits in Armenia and other countries.
APPLICATION
 The refractoriness and ductility of tungsten make it indispensable for incandescent filaments in lighting fixtures, as well as in picture tubes and other vacuum tubes.
The refractoriness and ductility of tungsten make it indispensable for incandescent filaments in lighting fixtures, as well as in picture tubes and other vacuum tubes.
Due to its high density, tungsten is the basis of heavy alloys that are used for counterweights, armor-piercing cores of sub-caliber and swept-finned artillery shells, armor-piercing bullet cores and high-speed gyroscope rotors to stabilize the flight of ballistic missiles (up to 180 thousand rpm).
Tungsten is used as electrodes for argon-arc welding. Alloys containing tungsten are characterized by heat resistance, acid resistance, hardness and abrasion resistance. They are used to make surgical instruments (amaloy alloy), tank armor, shells of torpedoes and shells, the most important parts of aircraft and engines, and containers for storing radioactive substances. Tungsten is an important component of the best grades of tool steels. Tungsten is used in high-temperature vacuum resistance furnaces as heating elements. An alloy of tungsten and rhenium is used in such furnaces as a thermocouple.
For mechanical processing of metals and non-metallic structural materials in mechanical engineering (turning, milling, planing, chiselling), well drilling, and in the mining industry, hard alloys and composite materials based on tungsten carbide are widely used (for example, pobedit, consisting of WC crystals in a cobalt matrix; grades widely used in Russia - VK2, VK4, VK6, VK8, VK15, VK25, T5K10, T15K6, T30K4), as well as mixtures of tungsten carbide, titanium carbide, tantalum carbide (TT grades for particularly difficult processing conditions, for example, chiselling and planing forgings made of heat-resistant steels and rotary hammer drilling of strong materials). Widely used as an alloying element (often together with molybdenum) in steels and iron-based alloys. High-alloy steel, classified as “high-speed”, with a marking starting with the letter P, almost always contains tungsten. (P18, P6M5. from rapid - fast, speed).
Tungsten sulfide WS 2 is used as a high-temperature (up to 500 °C) lubricant. Some tungsten compounds are used as catalysts and pigments. Tungstate single crystals (lead, cadmium, calcium tungstates) are used as scintillation detectors of X-rays and other ionizing radiation in nuclear physics and nuclear medicine.
Tungsten ditelluride WTe 2 is used to convert thermal energy into electrical energy (thermo-emf about 57 μV/K). The artificial radionuclide 185 W is used as a radioactive tracer in substance research. Stable 184 W is used as a component of alloys with uranium-235 used in solid-phase nuclear rocket engines, since it is the only common tungsten isotope that has a low thermal neutron capture cross section (about 2 barn).
Tungsten - W
CLASSIFICATION
| Nickel-Strunz (10th edition) | 1.AE.05 |
| Dana (7th edition) | 1.1.38.1 |
Introduction
The importance of rare elements in science and technology increases every year, and the boundary between rare and non-rare elements is increasingly blurred. A modern analytical chemist more and more often has to deal with the determination of tungsten, molybdenum, vanadium, titanium, zirconium and other rare elements.
Analysis of a mixture of all elements is an extremely rare case.
The many combinations of rare and non-rare elements found in minerals are so complex that analysis requires extensive experience and knowledge of rare element chemistry.
To separate elements into groups or to isolate any one element, not only precipitation reactions are used, but also other methods, such as: extraction of compounds with organic solvents, distillation of volatile compounds, electrolysis, etc.
Due to the difficulty of separating and determining some rare elements by chemical methods, these determinations are made by physical methods (spectral, luminescent, etc.).
When very small quantities of trace elements are detected, chemical enrichment methods are used, based on the coprecipitation of the element being determined with another specially selected element - the “carrier”. The carrier elements are selected in such a way as not to interfere with the further course of the analysis.
One of the most important rare elements is tungsten. In this paper we want to consider some issues related to the qualitative detection of tungsten.
History of the discovery of tungsten
The word "tungsten" existed long before the discovery of this metal. Even the German physician and metallurgist Georgius Agricola (1494-1555) called some metals tungsten. The word "tungsten" had many shades of meaning; it, in particular, meant both “wolf saliva” and “wolf foam”, i.e. foam at the mouth of an angry wolf. Metallurgists of the 14th-16th centuries noticed that when smelting tin, an admixture of some mineral causes significant losses of metal, turning it “into foam” - into slag. The harmful impurity was the mineral wolframite (Mn, Fe)WO4, similar in appearance to tin ore - cassiterite (SnO2). Medieval metallurgists called wolframite "tungsten" and said that "it steals and devours tin, like a wolf a sheep."
Tungsten was first obtained by the Spanish chemists the de Elujar brothers in 1783. Even earlier - in 1781. - Swedish chemist Scheele isolated tungsten trioxide WO3 from a mineral with the composition CaWO4, which later became known as “scheelite”. Therefore, tungsten was called sheelium for a long time.
In England, France and the USA, tungsten is called differently - tungsten, which means “heavy stone” in Swedish. In Russia in the 19th century, tungsten was called “thistle.”
Position in the periodic table of chemical elements
Tungsten is an element of group VI of the periodic system of chemical elements, its serial number is 74, atomic mass is 183.85.
Natural tungsten consists of a mixture of stable isotopes with masses:
Radioactive isotopes with masses from 174 to 188 are also known for tungsten.
Physicochemical properties of tungsten and its application
tungsten chemical qualitative detection
Pure metal tungsten is a silver-white metal, similar in appearance to steel, with a body-centered cubic crystal lattice; in powder form - dark gray in color.
Physical constants of tungsten:
Melting point. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3380-3430oC
Boiling point. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5900oC
Density (at 20 oC) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .19.3 g/cm3
Specific heat capacity (at 20 oC) . . . . . . . . . . . . . . . . . .0.032 cal/g* oC
Heat of fusion. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44 cal/g
Heat of vaporization. . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . .1.83 cal/g
The vapor pressure of tungsten is listed in Table 1 (see Appendix).
Tungsten has the highest melting point and lowest vapor pressure of any metal. Tungsten wire has the highest tensile strength and yield strength up to 420 kg/mm2.
Today, tungsten is widely used in science and technology. It is used for alloying steel, as a basis for superhard alloys, as a component of heat-resistant alloys for aviation and rocket technology, for the manufacture of cathodes of electric vacuum devices and filaments of incandescent lamps. Tungsten alloys have high heat resistance (at 16500C the ultimate strength is 175-253 MPa), however, they are brittle and above 6000C they oxidize intensively in air (without a protective coating they can only be used in a vacuum and a reducing or neutral atmosphere). They absorb ionizing radiation well. They are used for the manufacture of heating elements, heat shields, containers for storing radioactive drugs, thermal emitters, thermocouple electrodes used to measure temperatures up to 25000C (alloys with rhenium).
Chemical properties
Tungsten is one of the most corrosion-resistant metals. At normal temperatures it is resistant to water and air, at temperatures of 400-500 oC it noticeably oxidizes, at higher temperatures it oxidizes intensively, forming yellow tungsten trioxide. It does not interact with hydrogen even at very high temperatures; it interacts with nitrogen at temperatures above 2000 oC, forming nitride WN2. Solid carbon at 1100-1200 oC reacts with tungsten, forming carbides WC and W2C. In the cold, sulfuric, hydrochloric, nitric, hydrofluoric acids and aqua regia have no effect on tungsten. At a temperature of 100 oC, tungsten does not interact with hydrofluoric acid, weakly interacts with hydrochloric and sulfuric acids, and reacts more quickly with nitric acid and aqua regia. Quickly dissolves in a mixture of hydrofluoric and nitric acids. Alkali solutions in the cold have no effect on tungsten; molten alkalis in the presence of air or in the presence of oxidizing agents (such as nitrates, chlorates, lead dioxide) intensively dissolve tungsten, forming salts.
The distribution of electrons in a tungsten atom is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 5d4 6s2. Tungsten ionization potentials: I1=7.98eV; I2=17.7eV. Atomic radius rme=1.40Ao.
Ionic radii:
In compounds, tungsten exhibits oxidation states +2, +3, +4, +5, +6. In higher oxidation states, tungsten has acidic properties, in lower states it has basic properties. Compounds with oxidation state +2, +3 are unstable. Divalent tungsten is known only in the form of halides. Stable complex cyanides have been isolated from tungsten(IV) compounds in solid form. Tungsten(V) and (VI) compounds are of greatest practical importance in analysis.
The behavior of tungsten in solutions is complex, especially in acidic ones, due to the absence of simple compounds. Of significant importance in the analytical chemistry of tungsten is its great tendency to form complexes. Due to the fact that in complex compounds the individual properties of individual elements appear more clearly than in simple ones, complexation of tungsten is widely used in determination in the presence of elements with similar properties.
Tungsten(II) and (III) compounds are strong reducing agents; the oxidizing ability of tungsten(V) compounds is weak.
Thermodynamic data for tungsten and its compounds are given in Table 2 (see Appendix)
Until the 40s of the 20th century, the analytical chemistry of tungsten developed along with the analytical chemistry of molybdenum, and the former was characterized by gravimetric methods of determination. In recent years, the chemistry of tungsten coordination compounds has been successfully studied, some of which are successfully used in analytical chemistry for the determination of tungsten by physical and physicochemical methods.
The similarity of the properties of tungsten and molybdenum explains the difficulty of their separation and determination in the presence of each other. However, the difference in the distribution of valence electrons and the phenomenon of lanthanide contraction experienced by the electron shell of tungsten lead to differences in some of the chemical properties of these elements. For example, the tendency of aqueous solutions of tungsten(VI) to polymerize and hydrolyze in the presence of mineral acids is stronger than that of molybdenum(VI). Tungsten is more difficult to restore to certain lower oxidation states, the stabilization of which, unlike molybdenum, is complex and not always successful.
High-quality tungsten detection
Tungsten chemistry is extremely complex. Possessing a variable oxidation state, this element forms a large number of compounds. Here we will consider the properties of only those tungsten compounds that it forms when its alloys are dissolved in acids. Since concentrated nitric acid mixed with 2N is used to dissolve these alloys. sulfuric acid or aqua regia, tungsten goes into its highest oxidation state +6. Therefore, we will focus on the properties of tungsten(VI) compounds.
Partial reactions of the WO42- ion:
1. Acids. When solutions of tungstates are exposed to concentrated mineral acids, such as hydrochloric acid, a white precipitate of tungstic acid precipitates:
WO42-+2H++H2O = WO3*2 H2O.
When boiling, WO3*2 H2O turns into yellow WO3* H2O. Tungstic acid is insoluble in concentrated acids (unlike MoO3*H2O). The reaction of its formation is used to separate WO42- from other ions.
2. Hydrogen sulfide H2S in an acidic solution does not precipitate WO42-.
3. Ammonium sulfide (NH4)2S forms water-soluble thiosalts with tungstates, for example:
WO42- + 8NH4+ +4S2-+ 4 H2O = WS42- + 8NH4OH.
Upon acidification, the thiosal decomposes to form a light brown precipitate WS3.
4. Recovery of WO42-. A tungstate solution acidified with hydrochloric or sulfuric acid is treated with zinc metal. The initially formed precipitate of tungstic acid turns blue due to the formation of products of variable composition containing tungsten(VI) and (V) compounds:
Zn + 2WO42-+6H+ = W2O5+Zn2++3H2O.
The same compound is obtained by replacing zinc with a solution of tin(II) chloride.
In the hydrogen sulfide method of analysis, tungsten is classified as a subgroup of arsenic; however, it does not form sulfide under the action of hydrogen sulfide in an acidic environment, but forms it only under the action of ammonium and alkali metal sulfides or hydrogen sulfide in an alkaline environment; dissolves in excess sulfide to form a thiosalt:
Na2WO4 + 4 (NH4)2S + 4 H2O = Na2WS4 + 8 NH4OH.
When solutions of thiosalts are acidified, light brown tungsten sulfide precipitates:
Na2WS4 + 2 HCl = 2 NaCl + H2S + WS3,
dissolves in excess hydrochloric acid. But the WO42- ion is precipitated under the action of hydrochloric acid in the form of poorly soluble tungstic acid together with the silver group (Ag+, Hg22+, Tl(I), Pb2+) and is thus separated from most cations.
In the hydrogen sulfate-free analysis scheme, tungsten is also proposed to be isolated in the form of tungstic acid by the action of hydrochloric acid; together with it, the following ions are precipitated in the form of chlorides: Ag+, Hg22+, Tl (I), Pb2+. The systematic progress of the analysis of cations in the presence of tungsten is given in Table 3 (see Appendix).
Qualitative analysis of tungsten is very poorly developed. The precipitation of sparingly soluble tungstic acid by the action of mineral acids on tungstates is mainly used; Under these conditions, silicic acid is precipitated along with tungsten acid. Tungsten is separated from the latter by treating the precipitate with ammonia, and then found in the filtrate. Of the inorganic reagents, alkali metal and ammonium thiocyanates are most often used in the presence of titanium(III) and tin(II) reducing agents; of organic reagents, toluene-3,4-dithiol is used. It is likely that reagents recommended for the photometric determination of tungsten can be used for detection: they are sensitive and quite reliable, especially after separation of tungsten, for example, by acid hydrolysis. The reagents recommended for the gravimetric determination of tungsten are of little use for its detection, since they form uncharacteristic deposits with tungsten.
Korenman proposed detecting tungsten using ammonium chloride: colorless crystals of ammonium tungstate are shaped like diamonds and rods. Sensitivity 0.15 µg of tungsten in a drop of solution, maximum dilution 1:4 * 104. Detection is not interfered with by chlorides, sulfates, hundredfold amounts of molybdates and thirtyfold amounts of vanadates.
The rhodanide method makes it possible to detect by drop method 0.05-1% tungsten trioxide WO3 in ores and 10-4% tungsten in rocks.
Drip detection of tungsten in ores. The detection of 0.05-1% tungsten trioxide is not interfered with by 10% molybdenum and vanadium; 5% chromium; 2% each of arsenic and antimony, but it is recommended to separate vanadium and chromium.
About 5 mg of the sample, ground to powder, is fused with? 20 mg of sodium hydroxide, about 3 mg of sodium peroxide is added to the melt and melted again. The yellow color of the melt indicates the presence of chromium. A few drops of water are added to the melt, heated, transferred to a porcelain crucible and acidified with hydrochloric acid. The solution is evaporated in a water bath almost to dryness, the residue is moistened with hydrochloric acid, diluted with water, and filtered. The filter cake is treated with a hot ammonia solution (1:1), washed with hot water, the filtrate and washing water are combined and one drop of the reagent solution is added (30 g of potassium thiocyanate in 100 ml of water), evaporated to a small volume, 1-2 drops of concentrated hydrochloric acid are added acid, 1 drop of a 10% solution of tin(II) chloride and 1 drop of a 0.5% solution of titanium(III) chloride in hydrochloric acid (1:1). In the presence of tungsten, a yellow color appears.
Detection of tungsten in ores and rocks. Detection?1 10-4% of tungsten is interfered with by molybdenum, selenium, tellurium, large amounts of iron, vanadium, chromium, and silicon dioxide. Sulfide samples are fired and further crushed after firing.
0.5 g of finely ground substance is treated for 30 minutes in a test tube or microcup with 2 ml of hydrochloric acid while heating in a water bath. If arsenic is present, it is removed by the action of hydrazine in the presence of potassium bromide, evaporating the liquid after introducing the reagents to half the original volume. The residue is dissolved in two volumes of water, the solution is filtered through a cotton swab and washed with 1-2 ml of water. The filtrate and washing water are evaporated to dryness, dissolved in 1-2 drops of water, a 25% solution of potassium hydroxide is added dropwise until the iron hydroxide is completely precipitated, 3 drops of a saturated solution of ammonium thiocyanate are added, mixed, a 40% solution of tin(II) chloride is added until it disappears red coloring. In the presence of tungsten, a yellowish-green color appears.
To increase the sensitivity of detecting tungsten to 0.01 μg, it is recommended to perform the reaction on anion resin grains. Detection is not interfered with by 100-1000 μg of La, Ce(IV), Zr, Th, Mn, Fe, Ni, Zn, Cd, Al, Ga, In, Ge, Sn (IV), Pb, Sb (III), Bi, F-, Br-, I-, NO3-, SO32-, SO42-, HPO42-, B4O72-, HCOO-, C2O42-, citrate and tartrate. Pd, Pt, Ag, Au, Hg, As, Se, Te interfere.
In the presence of molybdenum, the solution is acidified with sulfuric acid to a concentration of 1-2M, molybdenum is extracted twice with a mixture of equal volumes of acetylacetone and chloroform, the aqueous layer is filtered, evaporated to a small volume, nitric acid is introduced to destroy organic substances, and sodium hydroxide is added to a concentration of 0.01M. The solution is placed on a white tile plate, several grains of Dauex-1-x-1 or 1-x-2 anion exchange resin are added, after a few minutes 1 drop of a 10% solution of tin(II) chloride in concentrated hydrochloric acid and a 3% solution of ammonium thiocyanate are added . In the presence of tungsten, the grain turns greenish. It is recommended to examine the grain under a microscope under a fluorescent lamp.
Drip detection of tungsten in steel. Kullberg proposes a reaction based on the ability of peroxotungstic acid, formed by the action of hydrogen peroxide on tungstic acid, to color an acetic acid solution of benzidine in an orange-red-brown color. The resulting compound is resistant to hydrogen peroxide.
A drop of an acid mixture (1 part 30% sulfuric acid and 1 part concentrated nitric acid) is placed on the cleaned steel surface. After 2-3 minutes, add a large excess of sodium peroxide, mix and add a 10% ammonia solution drop by drop until the boiling stops. Part of the sediment is captured with a piece of filter paper, and 2-3 drops of a freshly prepared 1% solution of benzidine in glacial acetic acid are placed on it. In the presence of tungsten, an orange-red-brown color appears.
In steels, tungsten can be detected by dithiol; molybdenum, zirconium, copper and other steel components do not interfere.
A 0.5-0.6 g sample of steel is dissolved in 10 ml of 6 M hydrochloric acid. Part of the solution is heated with tin(II) chloride to reduce molybdenum(VI) to molybdenum(III) and a methanol solution of dithiol is added. In the presence of tungsten, a bluish-green color appears.
When using rhodamine C, the detection sensitivity of tungsten is 0.001-0.0005 mg in 1 drop of solution. It is recommended to isolate tungstic acid H2WO4, then dissolve it in sodium hydroxide and detect tungsten in a slightly acidic environment. Detection without tungsten separation is interfered with by many ions, including I-, Br-, SCN-, Cr2O72-, S2O82-, MnO4-, ClO4-, S2O32- anions.
Rhodamine C is recommended for the detection of tungsten on paper chromatograms; to do this, they are sprayed with a 0.025% solution of rhodamine C in 1M sulfuric acid and a 20% solution of potassium bromide. The presence of tungsten can be identified by the color or luminescence of the spot.
When exposed to cathode or ultraviolet rays, scheelite luminesces intensely with blue light.
Properties of tungsten
Tungsten- it's metal. It is not found in sea water, not in the air, and in the earth’s crust it is only 0.0055%. That's how tungsten, element, standing in 74th position in. It was “opened” for industry by the World Exhibition in the French capital. It took place in 1900. The exhibition featured tungsten steel.
The composition was so hard that it could cut any material. remained “invincible” even at temperatures of thousands of degrees, which is why it was called red-resistant. Manufacturers from different countries who visited the exhibition adopted the development. The production of alloy steel has acquired a global scale.
Interestingly, the element itself was discovered back in the 18th century. In 1781, Swede Scheeler conducted experiments with the mineral tungsten. The chemist decided to place it in nitric acid. In the decomposition products, the scientist discovered an unknown gray metal with a silvery tint. The mineral on which experiments were carried out was later renamed scheelite, and the new element called tungsten.
However, it took a lot of time to study its properties, so worthy use of the metal was found much later. The name was chosen right away. The word tungsten existed before. The Spaniards called this one of the minerals found in the country's deposits.
The composition of the stone actually included element No. 74. Externally, the metal is porous, as if foamed. Therefore, another analogy came in handy. In German, tungsten literally means “wolf foam”.

The melting point of the metal rivals that of hydrogen, which is the most temperature-resistant element. Therefore, install tungsten softening index They couldn't for a hundred years. There were no furnaces capable of heating up to several thousand degrees.
When the “benefits” of the silver-gray element were “seen through,” they began to mine it on an industrial scale. For the 1900 exhibition, the metal was extracted the old fashioned way using nitric acid. However, tungsten is still mined this way.
Tungsten mining
Most often, the trioxide substance is first obtained from ore waste. It is processed at 700 degrees, obtaining pure metal in the form of dust. To soften the particles one has to resort to hydrogen. In it tungsten is melted down at three thousand degrees Celsius.
The alloy is used for cutters, pipe cutters, and milling cutters. for metal processing with using tungsten increase the accuracy of parts manufacturing. When exposed to metal surfaces, friction is high, which means that the working planes become very hot. Cutting and polishing machines without element No. 74 may themselves melt. This makes the cut inaccurate and imperfect.
Tungsten is not only difficult to melt, but also difficult to process. On the hardness scale, the metal occupies the ninth position. Corundum has the same number of points, the crumbs of which are used to make, for example, sandpaper. Only diamond is harder. Therefore, tungsten is processed with its help.
Applications of tungsten
The “steadfastness” of the 74th element attracts. Products made from alloys with gray-silver metal cannot be scratched, bent, or broken, unless, of course, you scratch them on the surface or with the same diamonds.

Tungsten jewelry has another undeniable advantage. They do not cause allergic reactions, unlike gold, silver, platinum and, even more so, their alloys with or. For jewelry, tungsten carbide is used, that is, its compound with carbon.
It is recognized as the hardest alloy in human history. Its polished surface perfectly reflects light. Jewelers call it “gray mirror”.
By the way, jewelry masters paid attention to tungsten after the cores of bullets, shells and plates for body armor began to be made from this substance in the mid-20th century.
Customer complaints about the fragility of high-grade silver jewelry forced jewelers to remember the new element and try to apply it in their industry. In addition, prices began to fluctuate. Tungsten has become an alternative to the yellow metal, which is no longer perceived as an investment item.
Being a precious metal, tungsten costs a lot of money. Per kilogram they ask for at least 50 dollars on the wholesale market. World industry spends 30 thousand tons of element No. 74 per year. More than 90% is absorbed by the metallurgical industry.

Only made from tungsten containers for storing nuclear waste. Metal does not transmit destructive rays. The rare element is added to alloys for making surgical instruments.
What is not used for metallurgical purposes is taken by the chemical industry. Tungsten compounds with phosphorus, for example, are the basis of varnishes and paints. They do not collapse or fade from sunlight.
A sodium tungstate solution resistant to moisture and fire. It becomes clear what waterproof and fireproof fabrics for divers’ and firefighters’ suits are impregnated with.
Tungsten deposits
There are several tungsten deposits in Russia. They are located in Altai, the Far East, the North Caucasus, Chukotka and Buryatia. Outside the country, the metal is mined in Australia, the USA, Bolivia, Portugal, South Korea and China.
In the Celestial Empire there is even a legend about a young explorer who came to China to look for a tin stone. The student settled in one of the houses in Beijing.

After a fruitless search, the guy loved to listen to the stories of the owner’s daughter. One evening she told the story of the dark stones from which the home stove was built. It turned out that the blocks were falling from the cliff into the backyard of the building. So, the student didn’t find it, but he did find tungsten.
With atomic number 74 in the periodic table, designated by the symbol W (Latin: Wolframium), it is a solid gray transition metal. The main application is as a basis for refractory materials in metallurgy. Extremely refractory, chemically resistant under standard conditions.
History and origin of the name
The name Wolframium was transferred to the element from the mineral wolframite, known back in the 16th century. called "wolf's foam" - "Spuma lupi" in Latin, or "Wolf Rahm" in German. The name was due to the fact that tungsten, accompanying tin ores, interfered with the smelting of tin, turning it into foam of slag (“tin devours like a wolf devouring a sheep”).
Currently, in the USA, Great Britain and France, the name “tungsten” (Swedish: tung sten - “heavy stone”) is used for tungsten.
In 1781, the famous Swedish chemist Scheele, treating the mineral scheelite with nitric acid, obtained a yellow “heavy stone”. In 1783, the Spanish chemists the Eluard brothers reported obtaining yellow oxide of a new metal, soluble in ammonia, from the Saxon mineral wolframite. Moreover, one of the brothers, Fausto, was in Sweden in 1781 and communicated with Scheele. Scheele did not lay claim to the discovery of tungsten, and the Eluard brothers did not insist on their priority.
Receipt
The process of obtaining tungsten goes through the substage of separation of trioxide WO 3 from ore concentrates and subsequent reduction to metal powder with hydrogen at a temperature of approx. 700 °C. Due to the high melting point of tungsten, powder metallurgy methods are used to obtain a compact shape: the resulting powder is pressed, sintered in a hydrogen atmosphere at a temperature of 1200-1300 °C, then an electric current is passed through it. The metal is heated to 3000 °C, and sintering occurs into a monolithic material. For subsequent purification and obtaining a single-crystalline form, zone melting is used.
Properties
Physical
Tungsten is a light gray metal that has the highest proven melting and boiling points (it is assumed that seaborgium is even more refractory, but so far this cannot be firmly stated - the lifetime of seaborgium is very short).
Tungsten is one of the heaviest, hardest and most refractory metals. In its pure form, it is a silver-white metal, similar to platinum, at a temperature of about 1600 ° C it is easily forged and can be drawn into a thin thread.
Chemical
Valence from 2 to 6. The most stable is 6-valent tungsten. 3- and 2-valent tungsten compounds are unstable and have no practical significance.
Tungsten has high corrosion resistance: at room temperature it does not change in air; at red-hot temperatures it slowly oxidizes into tungsten VI oxide; almost insoluble in hydrochloric, sulfuric and hydrofluoric acids. In nitric acid and aqua regia it oxidizes from the surface. It dissolves in a mixture of nitric and hydrofluoric acid, forming tungstic acid. Of the tungsten compounds, the most important are: tungsten trioxide or tungsten anhydride, tungstates, peroxide compounds with the general formula Me 2 WO x, as well as compounds with halogens, sulfur and carbon. Tungstates are prone to the formation of polymer anions, including heteropolycompounds with the inclusion of other transition metals.