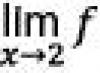Assignments. 1. Get ethylene from ethyl alcohol.
2. Carry out characteristic reactions for ethylene as a representative of unsaturated hydrocarbons.
Equipment. A device for producing ethylene, a stand with test tubes, glass tubes with an extended end, a splinter, a porcelain plate or cup, a cup of sand, a laboratory stand, a burner, matches, a beaker, a coiled copper wire, which should be inserted into the gas outlet tube.
Substances. Ethanol, sulfuric acid(conc.), a solution of bromine water and a pink solution of acidified potassium permanganate, washed and calcined river sand.
Getting the job done
1. Production of ethylene. Assemble the device for producing ethylene (Fig. 22.6) and check it for leaks.
To obtain ethylene, place 1.5 ml of ethyl alcohol in a test tube, then carefully pour in 4 ml of concentrated sulfuric acid and add a little calcined sand to the mixture. Close the test tube with a stopper with a gas outlet tube and secure the device in a stand.
2. Pour 2 ml of solutions of bromine water and potassium permanganate into two test tubes. Heat the mixture to a boil in a device for producing ethylene and, without ceasing to heat, but without overheating, lower the end of the gas outlet tube first into
test tube with bromine water, and then into a test tube with a solution of potassium permanganate.

What are you observing? Make up equations for chemical reactions: a) producing ethylene from ethyl alcohol; b) interaction of ethylene with bromine water.
Point the end of the gas outlet tube of the device upward and ignite the ethylene released with a torch. Note the nature of the flame. Place a porcelain plate or bowl into the ethylene flame for a few seconds. What are you observing?
Blow air through a glass tube with the end extended into the middle of the ethylene flame. How does the brightness of the flame change? Why? Write an equation for the combustion reaction of ethylene.
Production of ethylene.
Concentrated sulfuric acid has the property of taking water away from other substances. This property is used to produce ethylene.

The water partially condenses on the walls of the test tube and rolls back into the solution. Ethylene escapes through a gaseous tube. Properties of ethylene.
Chemistry lesson in 10th grade
Practical work № 2.
"Preparation of ethylene and experiments with it."
Target: consolidate students' knowledge on the topic “Alkanes. Alkenes”, teach how to produce ethylene and conduct experiments with it; improve the ability to receive gaseous substances in the simplest devices, observing safety regulations.
Planned learning outcomes: be able to conduct experiments on the production of ethylene and study its properties, observe safety rules when working with substances, equipment and chemical utensils, and write a report on practical work.
Equipment: on the students' tables: a laboratory stand with a foot, an alcohol lamp, matches, test tubes in a stand, a gas outlet tube, sand.
Reagents: bromine water, potassium permanganate solution, ethyl alcohol, concentrated sulfuric acid.
Lesson type: laboratory-practical.
Lesson structure.
I . Repetition of learned material.
1. Safety briefing against signature.
Then together we analyze the progress of practical work point by point, stopping
in detail on extreme caution when carrying out practical work.
2. Students begin to draw up practical work in notebooks for
practical work: write down the number, topic, purpose, equipment.
3. Then they do practical work. An issued test tube with a ready-made
a mixture of ethyl alcohol (2 - 3 ml), concentrated sulfuric acid
(6 - 9 ml) and calcined sand, close with a gas outlet tube, strengthen
in a laboratory stand and begin to heat it carefully, starting with warming up
the entire test tube.
a) C 2 H 5 OH → H 2 C = CH 2 + H 2 O
ethyl alcohol ethylene
The end of the gas outlet tube is lowered into a test tube into which 2-3 ml is poured
bromine water. After some time, the released gas discolors
bromine water. This means that a chemical reaction occurred and formed
new substance:
b) H 2 C = CH 2 + Br 2 → CH 2 Br – CH 2 Br
ethylene 1,2 – dibromoethane
4. After the bromine water has become colorless, pour 2-3 ml into another test tube
dilute solution of potassium permanganate acidified with sulfuric acid,
and also pass the resulting gas through it. After a while
the color disappears, the solution becomes transparent, which means also here
a chemical reaction occurred and a new substance was formed:
H 2 C = CH 2 + [O] + H 2 O → CH 2 – CH 2
ethylene ׀ ׀
ethylene glycol
5. After the experiments have been completed, remove the gas outlet tube from the test tube and
Set the released gas on fire, it burns with a luminous flame. Ethylene, like everyone else
hydrocarbons burn to form carbon dioxide and water:
C 2 H 4 +3O 2 → 2CO 2 + 2H 2 O
6. After finishing work, clean up the desktop and begin
designing the work in a notebook: describe the entire progress of the work, sketch
Figure 19 on page 56, as you work write the equations of the corresponding
reactions, at the end of the work draw a conclusion, while answering all questions for
independent conclusions; at the end of the lesson, notebooks are submitted for checking.
II . Homework.
repeat § 9 – 10.
Naturally - mathematical direction /
Goal: identifying the level of practical knowledge of students.
Know the laboratory method for producing ethylene, safety rules during work
With organic substances and concentrated acids;
Be able to practically produce ethylene, prove its unsaturated
properties characteristic reactions, make up equations
chemical reaction data;
Develop the ability to work in GPS/permanent groups/,
observe, compare, generalize, draw conclusions. Lesson type: workshop lesson
Form of training: GPS - when performing, individual - when documenting the results of the work.
Teaching methods: visual and practical, partially exploratory, independent work students.
Teaching aids: textbook “Chemistry-11” /EMN/, interactive board,
video stories, scheme-algorithm for practical work, a set of reagents and chemical glassware.
Lesson progress:
I Motivation / introductory remarks teachers/
Chemists are people who explore the world with their own hands, and to put it more scientifically, the main method of their work is experimental. There are many available and interesting experiments, which are suitable for beginner chemists. When performing them, you need to remember that even the simplest chemical experiment requires a very serious attitude: it must be staged competently, correctly and necessarily carried out in compliance with all the rules.
You, school graduates, study in a natural science class - mathematical direction. After graduating from school, one of you will definitely directly connect your life with chemistry, and if not, then you will indirectly come into contact with it, working in industries, medicine and other fields. Chemical knowledge will definitely come in handy, because they are an attribute of an educated, comprehensive - developed person, and by performing a chemical experiment in chemistry lessons, you will acquire such important future life qualities such as attentiveness, observation, accuracy and responsibility.
II Report the topic, purpose and objectives of the lesson.
III Recording in a notebook for practical work the topics, goals, reagents and equipment necessary for operation.
IV Preparation for practical work, updating students’ knowledge of knowledge:
Watch video stories /“Production of ethylene, interaction with a solution of potassium permanganate, interaction with bromine water, combustion”/
Conversation about the features and progress of practical work:
a) name the main laboratory method for producing ethylene;
b) what is the role of calcined sand or porcelain pieces when performing PR;
c) list the sequence of assembling the device for producing and collecting ethylene / the diagram of the device is shown on interactive whiteboard/;
Conversation about safety rules:
a) when collecting ethylene gas;
b) when checking the device for collecting ethylene for leaks;
c) when heating substances;
d) when working with concentrated sulfuric acid;
V. Drawing up a diagram - an algorithm for performing practical work
/ compiled by the teacher together with the students and projected on the interactive board - Appendix A /
VI. Execution of practical work / form of work - State Post Office, in each State Post Office there is a consultant who records in the control sheet the coefficient of participation of each group member when performing practical work - Appendix B/
VII. Lesson summary /teacher-appendix B/
Reflection / creative works students/
Mix acid and ethanol
Then we assembled the device.
If it is sealed,
Ethylene will turn out great. /A. Khamzina/
Discolored "potassium permanganate"
I managed to get bromine water,
Because he is infinite
C2H4 - ethylene gas / K. Faizulina/
We set fire to the ethylene, the torch became bright,
H2O and CO2 are the result of this fire. / O. Kirsch /
VIII. Homework. /Conduct a LO on the topic “ Unsaturated hydrocarbons"; “Home experiment in chemistry” M. Education, 1999, pp. 90-91/
Experiment No. 1 “Obtaining ethylene and studying its properties”
Clean river sand and clay from impurities, calcinate and dry. Fill one third of the test tube with this mixture and add three milliliters of cologne. Close the test tube with a stopper with a gas outlet tube and heat. Pass the released gas through a solution of potassium permanganate, then set it on fire. How to prove that the gas released is ethylene? How can we explain the chemistry of the production and interaction of ethylene with potassium permanganate?
Experience No. 2. The use of ethylene for fruit ripening.
Place two unripe tomatoes in two glass jars, if possible. same sizes. Cover the jars with pieces of cardboard, greasing the bottom edges. Pass ethylene into one jar, control into the second. Leave both jars for two days, then compare the results of the experiment in both jars. What is being observed? The same experiment can be done with unripe fruits pears and melons.
Appendix A
Scheme - algorithm for executing PR No. 2
“Preparation of ethylene and study of its properties”
Appendix B
Consultant checklist when performing PR No. 2
“Preparation of ethylene and experiments with it”
group No. 1
Khamzina A.
Appendix B
Teacher's control sheet when performing PR No. 2
“Preparation of ethylene and experiments with it”
Practical work No. 1
Obtaining ethylene and studying its properties
Repeat safety precautions when performing practical work!(sign in the safety notebook)
Purpose of the work: learn to produce ethylene in the laboratory and carry out high-quality reactions on unsaturated hydrocarbons of the ethylene series.
Work progress:
1. Pour 2-3 ml of ethyl alcohol into a test tube and carefully add 6-9 ml of concentrated sulfuric acid. Then add a little calcined sand (sand or small pieces of pumice are added to prevent the liquid from shaking when boiling). Close the test tube with a stopper with a gas outlet tube, secure it in a stand and carefully heat the contents of the test tube (Fig. 7, p. 44). What are you observing?
2. Pour 2-3 ml of bromine water into another test tube, lower the gas outlet tube to the bottom of this test tube and pass the released gas through the bromine water. What are you observing?
3. Pour 2-3 ml of a diluted solution of potassium permanganate into the third test tube and pass gas through it. What are you observing?
4. Taking the gas outlet tube out of the solution and turning it with the hole up, ignite the released gas. What kind of flame does ethylene burn with? Why?
5. Put out the alcohol lamp - the gas evolution will gradually stop.
Registration of completed work:
Write down the equations of the chemical reactions performed.
Conclusion:
When ethylene reacts with bromine water, the red-brown solution of bromine water becomes discolored. This reaction is quality to a double bond.
During ethylene oxidation aqueous solution Potassium permanganate produces ethylene glycol. It is noticeable that the purple color of the solution disappears. The reaction is quality to a double bond.
Unlike methane, ethylene burns with a luminous flame, which is due to its high carbon content.
Exercise:
A mixture of ethane and ethylene with a volume of 0.8 l (n.s.) decolorized 200 g of bromine water with mass fraction 1.6%. Determine the volume fraction of each gas in the mixture.
The goal of the work is to obtain ethylene and conduct experiments characterizing its properties. Equipment and reagents: alcohol lamp, matches, laboratory stand, stopper with gas outlet tube, stand with test tubes, filter paper; ethanol, river sand, H 2 SO 4 (conc.), bromine water, potassium permanganate solution.

Procedure 1. Pour 2 ml of ethyl alcohol into a test tube, carefully add 5 ml of concentrated sulfuric acid. Then add some river sand to prevent the liquid from boiling. Close the test tube with a stopper with a gas outlet tube, secure it in a stand. C 2 H 5 OH sand Watch video experiment


Procedure 3. To obtain ethylene, heat the mixture in a test tube to a boil. Continuing heating, lower the end of the gas outlet tube into a test tube with a solution of potassium permanganate (below the level of the solution). Pass the released ethylene through the potassium permanganate solution until the solution is completely discolored. Draw a conclusion about the reactivity of ethylene. Installation drawing


Procedure 4. Lower the end of the gas outlet tube into a test tube with bromine water (below the level of the solution). Pass the released ethylene through bromine water until completely decolorized. Draw a conclusion about the unsaturated nature of ethylene. Draw a conclusion about the reactivity of ethylene. Installation drawing 4.