Almost every day we all have to deal with one or another manifestation of the combustion process. In our article we want to tell in more detail what features this process includes from a scientific point of view.
It is the main component of the fire process. A fire begins with the occurrence of combustion, its development intensity is usually the path traveled by the fire, that is, the burning rate, and extinguishing ends with the cessation of combustion.
Combustion is usually understood as an exothermic reaction between a fuel and an oxidizer, accompanied by at least one of the following three factors: flame, glow, smoke formation. Due to the complexity of the combustion process, this definition is not exhaustive. It does not take into account such important features of combustion as the rapid occurrence of the underlying exothermic reaction, its self-sustaining nature and the ability of the process to self-propagate through the combustible mixture.
The difference between a slow exothermic redox reaction (iron corrosion, rotting) and combustion is that the latter occurs so quickly that heat is produced faster than it is dissipated. This leads to an increase in temperature in the reaction zone by hundreds and even thousands of degrees, to a visible glow and the formation of a flame. In essence, this is how flaming combustion is formed. If heat is released but there is no flame, then this process is called smoldering. In both processes, an aerosol of complete or incomplete combustion of substances occurs. It is worth noting that when some substances burn, the flame is not visible, and there is also no smoke emission; such substances include hydrogen. Too fast reactions (explosive transformation) are also not included in the concept of combustion.
A necessary condition for combustion to occur is the presence of a flammable substance, an oxidizer (in a fire, its role is played by oxygen in the air) and an ignition source. For direct combustion, critical conditions must exist in terms of the composition of the combustible mixture, the geometry and temperature of the combustible material, pressure, etc. After combustion occurs, the flame itself or the reaction zone acts as the ignition source.
For example, methane can be oxidized by oxygen with the release of heat to methyl alcohol and formic acid at 500-700 K. However, for the reaction to continue, it is necessary to replenish heat due to external heating. This is not combustion. When the reaction mixture is heated to a temperature above 1000 K, the rate of methane oxidation increases so much that the released heat becomes sufficient to further continue the reaction, the need for external heat supply disappears, and combustion begins. Thus, the combustion reaction, once it occurs, is capable of supporting itself. This is the main distinguishing feature of the combustion process. Another related feature is the ability of a flame, which is a chemical reaction zone, to spontaneously spread through a flammable medium or combustible material at a speed determined by the nature and composition of the reaction mixture, as well as the process conditions. This is the main mechanism of fire development.
A typical combustion model is based on the oxidation reaction of organic substances or carbon with atmospheric oxygen. Many physical and chemical processes accompany combustion. Physics is about the transfer of heat into a system. Oxidation and reduction reactions are a chemical component of the nature of combustion. Hence, from the concept of combustion, a variety of chemical transformations arise, including the decomposition of initial compounds, dissociation and ionization of products.
The combination of a flammable substance or material with an oxidizing agent constitutes a flammable medium. As a result of the decomposition of flammable substances under the influence of an ignition source, a gas-vapor-air reaction mixture is formed. Combustible mixtures, which in composition (ratio of fuel and oxidizer components) correspond to the equation of a chemical reaction, are called mixtures of stoichiometric composition. They are the most dangerous in terms of fire: they ignite more easily, burn more intensely, ensuring complete combustion of the substance, as a result of which they release the maximum amount of heat.
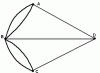
Rice. 1. Shapes of diffusion flames
a – burning of a jet stream, b – burning of a spilled liquid, c – burning of forest litter
Based on the ratio of the amount of combustible material and the volume of oxidizer, lean and rich mixtures are distinguished: poor mixtures contain an abundance of oxidizer, rich mixtures contain combustible material in abundance. The minimum amount of oxidizer required for complete combustion of a unit of mass (volume) of a particular combustible substance is determined by the equation of the chemical reaction. When burning with the participation of oxygen, the required (specific) air flow rate for most combustible substances is in the range of 4-15 m 3 /kg. Combustion of substances and materials is possible only when there is a certain content of their vapors or gaseous products in the air, as well as when the oxygen concentration is not lower than a specified limit.
So, for cardboard and cotton, self-extinguishing occurs already at 14 vol. % oxygen, and polyester wool - at 16 vol. %. In the combustion process, as in other chemical processes, two stages are required: the creation of molecular contact between the reagents and the very interaction of fuel molecules with the oxidizer to form reaction products. If the rate of transformation of the initial reagents is determined by diffusion processes, i.e. transfer rate (vapors of flammable gases and oxygen are transferred to the reaction zone due to a concentration gradient in accordance with Fick's laws of diffusion), then this combustion mode is called diffusion. In Fig. 1 shows various forms of diffusion flames. In the diffusion mode, the combustion zone is blurred, and a significant amount of incomplete combustion products is formed in it. If the combustion rate depends only on the rate of the chemical reaction, which is significantly higher than the rate of diffusion, then the combustion mode is called kinetic. It is characterized by higher combustion rates and completeness and, as a consequence, high heat release rates and flame temperatures. This regime occurs in pre-mixed mixtures of fuel and oxidizer. Hence, if the reagents in the chemical reaction zone are in the same (usually gas) phase, then such combustion is called homogeneous; when the fuel and oxidizer are in different phases in the reaction zone, it is called heterogeneous. The combustion of not only gases is homogeneous, but also most solids. This is explained by the fact that in the reaction zone it is not the materials themselves that burn, but their vapors and gaseous decomposition products. The presence of a flame is a hallmark of homogeneous combustion.
Examples of heterogeneous combustion are the combustion of carbon, carbonaceous wood residues, and non-volatile metals, which remain in a solid state even at high temperatures. The chemical combustion reaction in this case will occur at the interface between the phases (solid and gaseous). Note that the end products of combustion can be not only oxides, but also fluorides, chlorides, nitrides, sulfides, carbides, etc.
The characteristics of the combustion process are varied. They can be divided into the following groups: shape, size and structure of the flame; flame temperature, its emissivity; heat release and calorific value; burning rate and concentration limits of sustainable combustion, etc.
Everyone knows that combustion produces a glow that accompanies the combustion product.
Let's consider two systems:
- gaseous system
- condensed system
In the first case, when combustion occurs, the entire process will occur in the flame, while in the second case, part of the reactions will occur in the material itself or its surface. As mentioned above, there are gases that can burn without a flame, but if we consider solids, there are also groups of metals that are also capable of burning without a flame.
The part of the flame with the maximum value, where intense transformations occur, is called the flame front.
Heat exchange processes and diffusion of active particles from the combustion zone, which are the key mechanisms for the movement of the flame front through the combustible mixture.
The speed of flame propagation is usually divided into:
- deflagration (normal), occurring at subsonic speeds (0.05-50 m/s)
- detonation, when speeds reach 500-3000 m/s.

Rice. 2. Laminar diffusion flame
Depending on the nature of the speed of the gas flow creating the flame, laminar and turbulent flames are distinguished. In a laminar flame, the movement of gases occurs in different layers, all processes of heat and mass transfer occur through molecular diffusion and convection. In turbulent flames, the processes of heat and mass transfer are carried out mainly due to macroscopic vortex motion. A candle flame is an example of a laminar diffusion flame (Fig. 2). Any flame higher than 30 cm will already have random gas mechanical instability, which is manifested by visible swirls of smoke and flame.

Rice. 3. Transition from laminar to turbulent flow
A very clear example of the transition of a laminar flow to a turbulent one is a stream of cigarette smoke (Fig. 3), which, having risen to a height of about 30 cm, acquires turbulence.
During fires, flames have a diffusion turbulent character. The presence of turbulence in the flame enhances heat transfer, and mixing affects chemical processes. In a turbulent flame, the burning rate is also higher. This phenomenon makes it difficult to transfer the behavior of small-scale flames to large-scale flames with greater depth and height.
It has been experimentally proven that the combustion temperature of substances in air is much lower than the combustion temperature in an atmospheric oxygen environment
In air the temperature will fluctuate from 650 to 3100 °C, and in oxygen the temperature will increase by 500-800 °C.
Combustion
Combustion- a complex physical and chemical process of converting the components of a combustible mixture into combustion products with the release of thermal radiation, light and radiant energy. The nature of combustion can be described as rapidly occurring oxidation.
Subsonic combustion (deflagration), unlike explosion and detonation, occurs at low speeds and is not associated with the formation of a shock wave. Subsonic combustion includes normal laminar and turbulent flame propagation, and supersonic combustion includes detonation.
Combustion is divided into thermal And chain. At the core thermal Combustion is a chemical reaction that can proceed with progressive self-acceleration due to the accumulation of released heat. Chain combustion occurs in cases of some gas-phase reactions at low pressures.
Conditions for thermal self-acceleration can be provided for all reactions with sufficiently large thermal effects and activation energies.
Combustion can begin spontaneously as a result of self-ignition or be initiated by ignition. Under fixed external conditions, continuous combustion can occur in stationary mode, when the main characteristics of the process - reaction rate, heat release power, temperature and composition of products - do not change over time, or periodic mode when these characteristics fluctuate around their average values. Due to the strong nonlinear dependence of the reaction rate on temperature, combustion is highly sensitive to external conditions. This same property of combustion determines the existence of several stationary modes under the same conditions (hysteresis effect).
The combustion process is divided into several types: flash, combustion, ignition, spontaneous combustion, spontaneous ignition, explosion and detonation. In addition, there are special types of combustion: smoldering and cold-flame combustion. Flash is the process of instantaneous combustion of vapors of flammable and combustible liquids caused by direct exposure to an ignition source. Combustion is the phenomenon of combustion occurring under the influence of an ignition source. Ignition is a fire accompanied by the appearance of a flame. At the same time, the rest of the mass of the combustible substance remains relatively cold. Spontaneous combustion is a phenomenon of a sharp increase in the rate of exothermic reactions in a substance, leading to combustion in the absence of an ignition source. Spontaneous combustion is spontaneous combustion accompanied by the appearance of a flame. Under industrial conditions, sawdust and oily rags can spontaneously ignite. Gasoline and kerosene can ignite spontaneously. Explosion is a rapid chemical transformation of a substance (explosive combustion), accompanied by the release of energy and the formation of compressed gases capable of producing mechanical work.
Flameless burning
Unlike conventional combustion, when zones of oxidizing flame and reducing flame are observed, it is possible to create conditions for flameless combustion. An example is the catalytic oxidation of organic substances on the surface of a suitable catalyst, such as the oxidation of ethanol on platinum black.
Solid phase combustion
These are autowave exothermic processes in mixtures of inorganic and organic powders, not accompanied by noticeable gas evolution, and leading to the production of exclusively condensed products. Gas and liquid phases are formed as intermediate substances that provide mass transfer, but do not leave the burning system. There are known examples of reacting powders in which the formation of such phases has not been proven (tantalum-carbon).
The trivial terms “gasless combustion” and “solid flame combustion” are used synonymously.
An example of such processes is SHS (self-propagating high-temperature synthesis) in inorganic and organic mixtures.
Smoldering
A type of combustion in which no flame is formed, and the combustion zone slowly spreads throughout the material. Smoldering typically occurs in porous or fibrous materials that have a high air content or are impregnated with oxidizing agents.
Autogenous combustion
Self-sustaining combustion. The term is used in waste incineration technologies. The possibility of autogenous (self-sustaining) combustion of waste is determined by the maximum content of ballasting components: moisture and ash. Based on many years of research, the Swedish scientist Tanner proposed to use a triangle diagram with limiting values to determine the boundaries of autogenous combustion: more than 25% combustible, less than 50% moisture, less than 60% ash.
See also
Notes
Links
Wikimedia Foundation. 2010.
Synonyms:See what “Combustion” is in other dictionaries:
A physical and chemical process in which the transformation of a substance is accompanied by intense release of energy and heat and mass exchange with the environment. Combustion can begin spontaneously as a result of self-ignition or be initiated... ... Big Encyclopedic Dictionary
BURNING, burning, many. no, cf. (book). Action and condition according to Ch. burn. Gas burning. Mental burning. Ushakov's explanatory dictionary. D.N. Ushakov. 1935 1940 ... Ushakov's Explanatory Dictionary
Shine, play, enthusiasm, radiance, play, take off, elation, uplifting spirit, sparkle, sparkle, obsession, fire, passion, twinkle, inspiration, sparkle, inspiration, passion, zest, fascination, combustion, rise Dictionary... ... Dictionary of synonyms
Combustion- COMBUSTION, a chemical transformation that is accompanied by intense release of heat and heat and mass transfer with the environment. May start spontaneously (spontaneous combustion) or as a result of ignition. The characteristic property of combustion is the ability... ... Illustrated Encyclopedic Dictionary
Complex chemistry a reaction occurring under conditions of progressive self-acceleration associated with the accumulation of heat or catalyzing reaction products in the system. With G., high temperatures (up to several thousand K) can be achieved, and often occurs... ... Physical encyclopedia
A physical and chemical process in which the transformation of a substance is accompanied by intense release of energy and heat and mass transfer with the environment. may begin spontaneously as a result of self-ignition or may be initiated by... ... Dictionary of emergency situations
Combustion is a chemical reaction of oxidation of fuel with oxygen, occurring relatively quickly in time and releasing a large amount of heat.
During the combustion process, combustion products are heated to high temperatures.
The general equation for the combustion of any hydrocarbon gas with oxygen is as follows:
Where m And n– respectively, the number of carbon and hydrogen atoms in the molecule
Q– thermal effect of the oxidation reaction.
Table 3.1 shows the combustion reactions of the main combustible gases with oxygen.
Combustion reactions of flammable gases with oxygen
Table 3.1
Table 3.1 shows the oxidation reactions of the most well-known flammable gases with oxygen. However, in real conditions, the oxidizer (oxygen) is supplied to the combustion zone not in pure form, but as part of the air. It is known that air mainly consists of two parts: oxygen and nitrogen. The air also contains small amounts of carbon dioxide CO 2, as well as rare gases. Considering their insignificant amount in the air, we neglect them.
Thus, if we take the volume of air as 100%, then the oxygen content will be 21% and nitrogen 79%. Therefore, at 1 m 3 air oxygen is 79/21 = 3.76 m 3 nitrogen, or 1 m 3 oxygen contained in 100/21 = 4.76 m 3 air.
Taking into account the above relationships, we can write down the general equation for the combustion of hydrocarbons with air:
Table 3.2 shows the equations for the reaction of combustion of flammable gases with air.
It should be noted that the equations given in tables 3.1 and 3.2 are stoichiometric, i.e. This is the ratio of combustible gas and oxidizer (oxygen, air), at which the theoretically required amount of oxidizer is supplied to the combustible gas. However, in the practice of gas combustion under real conditions, it is necessary to supply somewhat more oxidizer into the zone than follows from the stoichiometric equations. This is mainly due to imperfect mixing of the combustible gas and the oxidizer.
Equations of combustion reactions of flammable gases with air
Table 3.2
The ratio of the actual consumption of the oxidizer (oxygen or air) to the theoretically necessary one is called the excess air coefficient and is designated α , i.e.:
Where V d– actual air flow;
V t– theoretically required amount of air.
Table 3.3 shows the values of the theoretically required amount of oxidizer (oxygen and air), as well as the volume of combustion products during combustion of 1 m 3 gas and excess air coefficient equal to 1 ( a = 1).
Theoretically required amount of oxidizer and volume of combustion products during combustion 1 m 3 at α = 1
Table 3.3
In practical calculations, sometimes we do not know the chemical composition of gases, but only the heat of combustion is known. It is necessary to determine the theoretically required amount of air required for complete combustion 1 m 3 gas.
For this case there is an empirical formula by D.I. Mendeleev:
Where Q n– lower calorific value of gas, kJ/m 3 .
The equations for the combustion reactions of various gases with oxygen and air reflect only the relationship between fuel and oxidizer, and do not explain the mechanism of these reactions. In real conditions, the combustion process is much more complicated.
The modern theory of the mechanism of the kinetics of gas combustion reaction was developed by the Soviet scientist, academician N.N. Semenov. According to his theory, chain reactions of gas combustion occur in the flame of a gas-air mixture. As a result, intermediate unstable products are formed in the form of free radical atoms. In accordance with the theory of N.N. Semenov's reaction of combustion of hydrogen with oxygen is not simply a matter of combining two molecules of hydrogen and one of oxygen to form two molecules of water. During the interaction of these two gases, intermediate substances are first formed in the form of hydrogen and oxygen atoms, and free hydroxyl radicals OH are also formed.
To start the combustion process, it is necessary to somehow activate the combustible mixture. In other words, it is necessary to create conditions under which the reagents will have a large supply of energy. This energy reserve is necessary for the combustion process to occur. The above energy reserve can be created by heating the gas-air mixture to its ignition temperature. This energy, called activation energy, is necessary mainly to break the existing intermolecular bonds in the reactants.
During the combustion process, new bonds are continuously formed along with the destruction of old ones. When new bonds are formed, a significant release of energy occurs, while the breaking of old bonds is always accompanied by energy expenditure. Due to the fact that during the combustion process the energy that is released during the formation of new bonds is of great importance, compared with the energy expended on breaking old bonds, the total thermal effect remains positive.
The reaction of hydrogen with oxygen is the simplest and most studied. Therefore, let's look at this branched reaction with an example.
In accordance with the theory of N.N. Semenov, at the initial moment of the reaction, as a result of the activation energy and collision of hydrogen and oxygen molecules, two hydroxyl radicals OH are formed:
![]() . (3.5)
. (3.5)
The free hydrogen atom H, in turn, reacts with an oxygen molecule. As a result, a hydroxyl radical OH and a free oxygen atom are formed, i.e.:
![]() . (3.7)
. (3.7)
The radical can again enter into a chemical reaction with hydrogen and again, as a result of the reaction, form water and free hydrogen, and the oxygen atom, in turn, can react with a hydrogen molecule, which will lead to the formation of another OH radical and a hydrogen atom H , i.e.:
![]() . (3.8)
. (3.8)
The above mechanism of the chain reaction of combustion of hydrogen with oxygen shows the possibility of multiple interactions of one OH radical with hydrogen atoms. As a result of this interaction, water molecules are formed.
Therefore, free atoms and radicals are the active centers in creating a chain reaction.
The combustion reaction of hydrogen with oxygen, which explains the mechanism of the chain reaction, can be written as follows:
H 2 O O + (H 2)…
OH + (H 2) ® H + (O 2) ® OH + (H 2)…
O + (H 2) ® OH + (H 2) ® H 2 O
H +(O 2) ® OH +H 2 ...
The combustion mechanism of carbon monoxide with oxygen is more complex. According to scientists from the Institute of Chemical Physics of the USSR Academy of Sciences, carbon monoxide does not react with dry oxygen. They also found that adding a small amount of hydrogen or moisture to the mixture leads to the onset of an oxidation reaction. As a result, the following sequence of chemical reactions occurs:
H 2 O ® OH + H; (3.10)
OH + CO ® CO 2 + H; (3.11)
H + O 2 ® OH + O; (3.12)
CO + OH ® CO 2 + H; (3.13)
CO + O ® CO 2 ; (3.14)
H + O 2 = OH + O (3.15)
As follows from the above chemical reactions, the presence of a small amount of moisture leads to the formation of hydroxyls and free atoms in the combustion zone. As noted earlier, both hydroxyl radicals and free atoms are the initiators of the creation and carriers of the chain reaction.
An even more complex mechanism for the oxidation of hydrocarbons. Along with some similarities with the combustion mechanism of hydrogen and carbon monoxide, the combustion mechanism of hydrocarbons also has a number of significant differences. Analyzing the combustion products, it was found that they contain aldehydes and mainly formaldehyde (HCHO).
Let's consider the mechanism of hydrocarbon oxidation using the example of the simplest of them - methane. The mechanism of methane oxidation goes through four stages, at each of which the following chemical reactions occur:
At the first stage:
H + O 2 ® OH + O; (3.16)
CH 4 + OH ® CH 3 + H 2 O; (3.17)
CH 4 + O ® CH 2 + H 2 O. (3.18)
At the second stage:
CH 3 + O 2 ® HCHO + OH; (3.19)
CH 2 + O 2 ® HCHO + O; (3.20)
At the third stage:
HCHO + OH ® HCO + H 2 O (3.21)
HCHO + O ®СО + H 2 O; (3.22)
HCO+ O 2 ® CO + O + OH (3.23).
At the fourth stage:
CO + O ® CO 2 (3.24)
Combustion is an oxidation reaction that occurs at high speed, which is accompanied by the release of large amounts of heat and, as a rule, a bright glow, which we call a flame. The combustion process is studied by physical chemistry, in which all exothermic processes that have a self-accelerating reaction are considered to be combustion. Such self-acceleration can occur due to an increase in temperature (i.e., have a thermal mechanism) or the accumulation of active particles (have a diffusion nature).
The combustion reaction has a clear feature - the presence of a high-temperature region (flame), limited spatially, where most of the conversion of the starting substances (fuel) occurs. This process is accompanied by the release of a large amount. To start the reaction (the appearance of a flame), it is necessary to spend a certain amount of energy on ignition, then the process occurs spontaneously. Its speed depends on the chemical properties of the substances participating in the reaction, as well as on gas-dynamic processes during combustion. The combustion reaction has certain characteristics, the most important of which are the calorific value of the mixture and the temperature (called adiabatic) that could theoretically be achieved during complete combustion without taking into account heat loss.
Homogeneous combustion is the simplest, has a constant speed, depending on the composition and molecular thermal conductivity of the mixture, temperature and pressure.
Heterogeneous combustion is most common both in nature and in artificial conditions. Its speed depends on the specific conditions of the combustion process and on the physical characteristics of the ingredients. For liquid fuels, the rate of combustion is greatly influenced by the rate of evaporation, and for solid fuels - by the rate of gasification. For example, when burning coal, the process forms two stages. In the first of them (in the case of relatively slow heating) volatile components of the substance (coal) are released, in the second the coke residue burns out.
The combustion of gases (for example, the combustion of ethane) has its own characteristics. In a gaseous environment, flames can spread over a wide distance. It can move through a gas at subsonic speed, and this property is inherent not only in a gas environment, but also in a finely dispersed mixture of liquid and solid flammable particles mixed with an oxidizer. To ensure stable combustion in such cases, a special design of the furnace device is required.
The consequences caused by the combustion reaction in a gaseous environment are of two types. The first is turbulization of the gas flow, leading to a sharp increase in the speed of the process. The resulting acoustic disturbances of the flow can lead to the next stage - the emergence of a mixture leading to detonation. The transition of combustion to the detonation stage depends not only on the gas’s own properties, but also on the size of the system and propagation parameters.
Fuel combustion is used in technology and industry. The main task in this case is to achieve maximum combustion efficiency (i.e., optimization of heat release) for a given period. Combustion is used, for example, in mining - methods for developing various minerals are based on the use of a combustible process. But in certain natural and geological conditions, the phenomenon of combustion can become a factor that poses a serious danger. The real danger, for example, is the process of spontaneous combustion of peat, leading to the occurrence of endogenous fires.
Execution samples s/p2
CHEMICAL THERMODYNAMICS. EQUILIBRIUM. KINETICS.
TASK 1. Heat of combustion of fuel.
We have a gas fuel mixture: 50% CH 4 + 50% C 4 H 10.
Total volume V=1000 l=1m 3.
1. Write the chemical equations for the combustion reactions of the gas components of a given fuel mixture.
Methane combustion reaction:
CH 4 (g) + 2O 2 (g) ® CO 2 (g) + 2H 2 O (l)
Butane combustion reaction:
C 4 H 10 (g) + 13/2O 2 (g) ® 4СО 2 (g) + 5H 2 O (l).
Enthalpy Δ r N 0 298 of these chemical reactions is the heat of combustion of gas fuel Δ N 0 sg.
2. Calculate how much heat can be obtained by burning a given volume of a fuel mixture of a given composition (volume %), conditions considered normal.
Using Hess's law, we calculate the heat of combustion of gas fuel Δ N 0 сг at the standard state and 298 K, using tabular data (see appendix, table) of the heat of formation of all substances participating in the combustion reaction (Δ f N 0 298):
for methane
Δ N 0 сг СН4 = Δ r N 0 298 = Δ f N 0 CO2 + Δ f N 0 H2O - Δ f N 0 CH4 - 2Δ f N 0 O2 =
393.62 + 2. (-285.84) – (-74.78) - 0 = -802.28 kJ/mol.
for butane
Δ N 0 сг С4Н10 = Δ r N 0 298 = 4Δ f N 0 CO2 + 5Δ f N 0 H2O - Δ f N 0 С4Н10 - 13/2Δ f N 0 O2 =
4. (- 393.62) + 5 . (-285.84) – (-126.15) - 0 = -2877.53 kJ/mol.
Specific heat of combustion Q T of gas fuel:
Q T = - (Δ N sg. 1000/22.4), kJ/m 3,
where 22.4 l/mol is the molar volume of gas at normal conditions.
for methane
Q T, CH4 = - (-802.28. 1000 / 22.4) = 35816 kJ/m 3.
for butane
Q T, C4H10 = - (-2877.53. 1000 / 22.4) = 128461 kJ/m 3.
The total amount of heat obtained during the combustion of a given fuel mixture, taking into account the volume of gases:
Q = Q T, CH4 . V CH4 + Q T, С4Н10 . V С4Н10 =
35816. (1 . 0.5)+128461 . (1.0.5) =82138.5 kJ.
3. From the given fuel mixture, select the most energy efficient fuel. Calculate the specific heat of combustion of this fuel Q T , kJ/m 3 . Calculate the minimum volume of this fuel to obtain 100 MJ of heat.
The most energy efficient fuel in this fuel mixture is butane, specific heat of combustion Q T, C4H10 = 128461 kJ/m3.
To obtain 100 MJ of heat it is necessary to burn:
V С4Н10 = Q/ Q T, C4H10 = 100000/128461 = 0.778 m 3 = 778 l.
TASK 2. Chemical thermodynamics.
1. Write thermochemical equations of reactions, the thermal effect of which is the heat of formation of all reagents of a given chemical reaction.
For a chemical reaction
CO 2 (g) + C (k) « 2CO (g)
Substance C (k) is simple, stable at 298 K and a pressure of 100 kPa, its enthalpy of formation is D H 0 f , 298 , = 0.
Thermochemical equations of reactions, the thermal effect of which is the heat of formation of the reagents of a given chemical reaction CO 2 (g) and CO (g):
O 2 (g) + C (k) « CO 2 (g), D H 0 f , 298 = -393.51 kJ/mol,
(see table);
1/2 O 2 (g) + C (k) « CO (g) , D H 0 f , 298 = -110.5 kJ/mol,
(see table).
2. Calculate enthalpy valuesD r H 0 298 , entropyD r S 0 298 . table to problems 1, 2) at the standard state (s.s.) of all reagents and a temperature of 298 K. Draw a conclusion about the thermal effect of the reaction.
Using tabular data (see table), we write down the thermodynamic functions of the state of the reagents of a given chemical reaction at the standard state and 298 K
Using Hess's law, we calculate the enthalpy Δ r N 0 298, entropy ∆ r S 0 298 and Gibbs energy Δ r G 0 298 chemical reaction at standard state and 298 K:
Δ r N 0 298 = 2Δ f N 0 298 COg - Δ f N 0 298 Sk - Δ f N 0 298 CO2g =
2(-110.5) – 0 – (-393.5) = 172.5 kJ.
Δ r N 0 298 >0 - the reaction is endothermic and occurs with the absorption of heat.
∆ r S 0 298 = 2 S 0 f , 298.СО(g) - S 0 f , 298,С(к) - S 0 f , 298.СО2(g) = 2(197.54) – 5.74 – 213.68 =
175.66 J/K.
∆ r S 0 298 >0 – the system has become more disordered due to the formation of an additional amount of gas.
3. Calculate the value of the Gibbs energyD r G 0 298 a given chemical reaction (clause 1. table to problems 1, 2) at the standard state (s.s.) of all reagents and a temperature of 298 K. Determine in which direction this reaction will spontaneously proceed at the standard state of all reagents and a temperature of 298 K.
Δ r G 0 298 = 2Δ f G 0 298 COg - Δ f G 0 298 Sk - Δ f G 0 298 CO2g =
2(-137.14) – 0 – (-394.38) = 120.15 kJ.
Δ r G 0 298 >0 – spontaneous reaction in the forward direction at the standard state and 298 K is impossible. The reaction proceeds in the opposite direction.
4. Determine the temperature range at which spontaneous occurrence of a direct reaction is possible in the standard state of all reagents without taking into account the dependence D r H 0 AndD r S 0 on temperature. Plot the Gibbs energy of the reaction as a function of temperature.D r G 0 = f (T ).
The possibility of a spontaneous reaction under the standard state is determined by the inequality ∆ r G 0 T < 0.
Those. , If
∆ r
G 0
T
=
∆ r
H 0 298
+∆ r
With 0
p dT- T∆ r
S 0
298
-
T
 ∆ r
With 0
p /
T)dT
<
0
∆ r
With 0
p /
T)dT
<
0
∆ r G 0 T ≈ ∆ r H 0 298 - T∆ r S 0 298 < 0
∆ r G 0 T = (172,5 – T . 175,66 . 10 -3) < 0 , отсюда T> 982 K.
Dependency graph D r G 0 = f (T):
 ∆ r G 0
T
∆ r G 0
T
298 982 2300 T
Taking into account the temperature ranges of existence of reagents, the temperature range of spontaneous reaction in the standard state is 982< T< 2300 К.
5. Calculate the value of the Gibbs energyD r G 298 chemical reaction at given values of partial gas pressures (clause 2. table to problems 1, 2) and a temperature of 298 K. Determine whether the direction of the process at 298 K changes when the partial pressures of gases change compared to the standard state.
Calculation of the Gibbs energy of a chemical reaction at any temperature and any relative partial pressures of gases is carried out using the Van't Hoff isotherm equation:
Δ r G T =
∆
r G 0
T
+ RT ln  .
.
Let's calculate Δ r G 298 at 298 K and gas pressures: r CO = 2. 10 3 Pa,
r CO2 = 8. 10 5 Pa.
Relative partial pressures of gases:
 CO
= 2 . 10 3 Pa/10 5 Pa = 0.02;
CO
= 2 . 10 3 Pa/10 5 Pa = 0.02;
 CO2 = 8. 10 5 Pa/10 5 Pa = 8.
CO2 = 8. 10 5 Pa/10 5 Pa = 8.
Δ r G 298 = Δ r G 0 298 + RTln(r 2 CO / r CO2) = 120.15 +8.31. 10 -3. 298. ln(0,02/8) =
Δ r G 298 >0 – spontaneous reaction in the forward direction at given partial gas pressures and 298 K is impossible. The reaction proceeds in the opposite direction.
6. Determine how to (theoretically) change the partial pressure of any of the source gases (r A orr IN) to change the direction of the process compared to the standard state at 298 K and the standard partial pressure of all other components of the chemical reaction.
At the standard state and 298 K, the reaction may spontaneously occur in the opposite direction, because Δ r G 0 298 >0.
To change the direction of the process compared to the standard state at 298 K, you can change the partial pressure of CO 2 (the state of all other components is standard). The condition for spontaneous reaction in the forward direction is Δ r G 298 < 0.
According to the Van't Hoff isotherm equation:
Δ r G T =
∆
r G 0
T
+ RT ln  <
0
<
0
Δ r G 298
=
120.15 + 8.31. 10 -3. 298 ln  < 0
< 0
Solving the inequality ln
 <
- 48,5и
получаем:
<
- 48,5и
получаем:
 <
10 -21
.
<
10 -21
.
Thus, r CO< r CO2 ≈ 10 5 times.
Thus, to change the direction of the process compared to the standard state at 298 K and pressure r CO = 10 5 Pa, you need to increase the partial pressure of CO 2 by 10 5 times, i.e. partial pressure of CO 2 should be: r CO2 > 10 25 Pa.
At this CO 2 pressure, a given chemical reaction can spontaneously proceed in the forward direction at 298 K.
TASK 2. Chemical balance.
For a chemical reaction
CO 2 (g) + C (k) « 2CO (g)
1. Calculate the Gibbs energyD r G 0 T and equilibrium constantTO r of this reaction at the standard state and temperatures of 298 K, 500 K, 800 K, 1000 K, taking into account the dependenceD r H 0 T AndD r S 0 T on temperature at a constant specific heat capacity of substancesWith r = const . Plot the dependency graph
TO r = f (T ).
Let us calculate the change in the heat capacity of the system (∆ r c 0 r= const):
∆ r With 0 r = 2With 0 r 298COg – With 0 r 298Sk – With 0 r 298СО2g =
2. (29.14)–8.54–37.41 =12.33 J/K.
Let us calculate the Gibbs energy of a chemical reaction at the standard state and given temperatures of 298 K, 500 K, 800 K, 1000 K, taking into account the dependence ∆ r H 0 T and ∆ r S 0 T on temperature, considering the specific heat capacity of substances constant With r , according to the formula:
∆ r G 0 T = ∆ r H 0 T - T . ∆ r S 0 T = ∆ r G 0 298 + ∆ r With 0 r (T - 298) –T . ∆ r With 0 r ln(T / 298).
∆ r G 0 298 =120.15 kJ;
∆ r G 0 500 =120.15+12.33. 10 -3. (500-298) - 500. 12.33. 10 -3. ln(500/298)=
∆ r G 0 800 =120.15+12.33. 10 -3. (800-298) - 800. 12.33. 10 -3. ln(800/298)=
∆ r G 0 1000 =120.15+12.33. 10 -3. (1000-298) - 1000. 12.33. 10 -3. ln(1000/298) =
Thermodynamic condition for chemical equilibrium: ∆ r G T = 0.
Gibbs energy of a chemical reaction at the standard state
∆ r G 0 T related to the equilibrium constant TO r by ratio:
∆ r G 0 T = - RT ln TO r
Having calculated the value ∆ r G 0 T reaction, calculate the equilibrium constant TO r according to the formula:
K p= exp(- ∆G 0 T /RT) ,
Where R=8.31 J/mol. K is the universal gas constant.
K p, 298 = exp(- ∆G 0 T , 298 / R. 298) = exp(-120.15/8.31 . 10 -3. 298) = 8 . 10 -22;
K p, 500 = exp(- ∆G 0 T , 500 / R. 500) = exp(-84.67/8.31 . 10 -3. 500) = 1.4 . 10 -9 ;
K p, 800 = exp(- ∆G 0 T , 800 / R. 800) = exp(-31.97/8.31 . 10 -3. 800) = 8.1 . 10 -3 ;
K p, 1000 = exp(- ∆G 0 T , 1000 / R. 1000) = exp(3.16/8.31. 10 -3. 1000) = 1.46.
As the temperature increases, the equilibrium constant increases, which is explained by the endothermic thermal effect of this reaction
( Δ r N 0
T >0).
Δ r N 0
T >0).

2. Select any temperature from the region of spontaneous reaction in the forward direction. At this temperature, calculate the equilibrium concentrations of gaseous reagents if their initial concentrations were equal, respectively (see paragraph 3, table for problems 1,2).
At T=1000 K the reaction proceeds spontaneously in the forward direction, because ∆ r G 0 1000 = - 3.16 kJ<0, K p , 1000 = 1,46.
Let's choose the temperature T=1000 to calculate the equilibrium concentrations of gaseous reagents, if the initial concentrations of gaseous reagents CO 2 and CO were equal: With CO2 = 0.5 mol/l, With CO =0.
Expressions for equilibrium constants expressed in terms of relative equilibrium partial pressures of gases ( r equals ) and equilibrium concentrations ( With equal) :
TO r
=
 ;
TO With
=
;
TO With
=

K p And K With connected through the equation of gas state:
K With, 1000
=
 =
=
 =
0,018
=
0,018
Where R=0.082 l. atm/mol. K - universal gas constant;
∆ν = 2-1= 1 (change in the number of moles of gaseous substances during the reaction).
Material balance table:
We substitute the equilibrium concentrations of gaseous reagents into the expression for K With and solve the algebraic equation for X:
TO With
=
 = 0,018 , X= 0.0387mol/l
= 0,018 , X= 0.0387mol/l
WITH CO equal = 2. 0.0387 = 0.0774mol/l
WITH CO2equal = 0.5 - 0.0387 = 0.4613 mol/l.