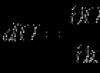Nuclear technologies are largely based on the use of radiochemistry methods, which in turn are based on the nuclear physical, physical, chemical and toxic properties of radioactive elements.
In this chapter we will limit ourselves to a brief description of the properties of the main fissile isotopes - uranium and plutonium.
Uranus
Uranus ( uranium) U - element of the actinide group, 7-0th period of the periodic table, Z=92, atomic mass 238.029; the heaviest found in nature.
There are 25 known isotopes of uranium, all of them radioactive. The easiest 217U (Tj/ 2 =26 ms), the heaviest 2 4 2 U (7 T J / 2 =i6.8 min). There are 6 nuclear isomers. Natural uranium contains three radioactive isotopes: 2 8 and (99, 2 739%, Ti/ 2 = 4.47109 l), 2 35 U (0.7205%, G, / 2 = 7.04-109 years) and 2 34 U ( 0.0056%, Ti/ 2=2.48-yuz l). The specific radioactivity of natural uranium is 2.48104 Bq, divided almost in half between 2 34 U and 288 U; 2 35U makes a small contribution (the specific activity of the 2 zi isotope in natural uranium is 21 times less than the activity of 2 3 8 U). Thermal neutron capture cross-sections are 46, 98 and 2.7 barn for 2 zzi, 2 35U and 2 3 8 U, respectively; division section 527 and 584 barn for 2 zzi and 2 z 8 and, respectively; natural mixture of isotopes (0.7% 235U) 4.2 barn.
Table 1. Nuclear physical properties 2 h9 Ri and 2 35Ts.
Table 2. Neutron capture 2 35Ts and 2 z 8 C.
Six isotopes of uranium are capable of spontaneous fission: 282 U, 2 zzi, 234 U, 235 U, 2 z 6 i and 2 z 8 i. Natural isotopes 2 33 and 2 35 U fission under the influence of both thermal and fast neutrons, and 2 3 8 nuclei are capable of fission only when capturing neutrons with an energy of more than 1.1 MeV. When capturing neutrons with lower energy, the 288 U nuclei first transform into 2 -i9U nuclei, which then undergo p-decay and transform first into 2 -"*9Np, and then into 2 39Pu. The effective cross sections for the capture of thermal neutrons of 2 34U, 2 nuclei 35U and 2 з 8 and are equal to 98, 683 and 2.7-barn, respectively. The complete division of 2 35U leads to a “thermal energy equivalent” of 2-107 kWh/kg. The isotopes 2 35U and 2 zi are used as nuclear fuel. fission chain reaction.
Nuclear reactors produce n artificial isotopes of uranium with mass numbers 227-^240, of which the longest-lived is 233U (7 V 2 =i.62 *io 5 years); it is obtained by neutron irradiation of thorium. In the super-powerful neutron fluxes of a thermonuclear explosion, uranium isotopes with mass numbers of 239^257 are born.
Uran-232- technogenic nuclide, a-emitter, T x / 2=68.9 years, parent isotopes 2 h 6 Pu(a), 23 2 Np(p*) and 23 2 Ra(p), daughter nuclide 228 Th. The intensity of spontaneous fission is 0.47 divisions/s kg.
Uranium-232 is formed as a result of the following decays:
P + -decay of nuclide *3 a Np (Ti/ 2 =14.7 min):
In the nuclear industry, 2 3 2 U is produced as a by-product during the synthesis of the fissile (weapon-grade) nuclide 2 zi in the thorium fuel cycle. When 2 3 2 Th is irradiated with neutrons, the main reaction occurs:
and a two-step side reaction:
The production of 232 U from thorium occurs only with fast neutrons (E„>6 MeV). If the starting substance contains 2 3°TH, then the formation of 2 3 2 U is complemented by the reaction: 2 3°TH + u-> 2 3'TH. This reaction occurs using thermal neutrons. Generation of 2 3 2 U is undesirable for a number of reasons. It is suppressed by using thorium with a minimum concentration of 2 3°TH.
The decay of 2 × 2 occurs in the following directions:
A decay in 228 Th (probability 10%, decay energy 5.414 MeV):
the energy of emitted alpha particles is 5.263 MeV (in 31.6% of cases) and 5.320 MeV (in 68.2% of cases).
- - spontaneous fission (probability less than ~ 12%);
- - cluster decay with the formation of nuclide 28 Mg (probability of decay less than 5*10" 12%):
Cluster decay with the formation of nuclide 2
Uranium-232 is the founder of a long decay chain, which includes nuclides - emitters of hard y-quanta:
^U-(3.64 days, a,y)-> 220 Rn-> (55.6 s, a)-> 21b Po->(0.155 s, a)-> 212 Pb->(10.64 hours , p, y) -> 212 Bi -> (60.6 m, p, y) -> 212 Po a, y) -> 208x1, 212 Po -> (3 "Yu' 7 s, a) -> 2o8 Pb (stab), 2o8 T1->(3.06 m, p, y-> 2o8 Pb.
The accumulation of 2 3 2 U is inevitable during the production of 2 zi in the thorium energy cycle. Intense y-radiation arising from the decay of 2 3 2 U hinders the development of thorium energy. What is unusual is that the even isotope 2 3 2 11 has a high fission cross section under the influence of neutrons (75 barns for thermal neutrons), as well as a high neutron capture cross section - 73 barns. 2 3 2 U is used in the radioactive tracer method in chemical research.
2 h 2 and is the founder of a long decay chain (according to the 2 h 2 T scheme), which includes nuclides emitters of hard y-quanta. The accumulation of 2 3 2 U is inevitable during the production of 2 zi in the thorium energy cycle. Intense y-radiation arising from the decay of 232 U hinders the development of thorium energy. What is unusual is that the even isotope 2 3 2 U has a high fission cross section under the influence of neutrons (75 barns for thermal neutrons), as well as a high neutron capture cross section - 73 barns. 2 3 2 U is often used in the radioactive tracer method in chemical and physical research.
Uran-233- man-made radionuclide, a-emitter (energy 4.824 (82.7%) and 4.783 MeV (14.9%)), Tvi= 1.585105 years, parent nuclides 2 37Pu(a)-? 2 33Np(p +)-> 2 ззРа(р), daughter nuclide 22 9Th. 2 zzi is obtained in nuclear reactors from thorium: 2 z 2 Th captures a neutron and turns into 2 zzT, which decays into 2 zzRa, and then into 2 zzi. The nuclei of 2 zi (odd isotope) are capable of both spontaneous fission and fission under the influence of neutrons of any energy, which makes it suitable for the production of both atomic weapons and reactor fuel. Effective fission cross section is 533 barn, capture cross section is 52 barn, neutron yield: per fission event - 2.54, per absorbed neutron - 2.31. The critical mass of 2 zzi is three times less than the critical mass of 2 35U (-16 kg). The intensity of spontaneous fission is 720 divisions/s kg.
Uranium-233 is formed as a result of the following decays:
- (3 + -decay of nuclide 2 33Np (7^=36.2 min):

On an industrial scale, 2 zi is obtained from 2 32Th by irradiation with neutrons:
When a neutron is absorbed, the 2 zzi nucleus usually splits, but occasionally captures a neutron, turning into 2 34U. Although 2 zzi usually divides after absorbing a neutron, it sometimes retains a neutron, turning into 2 34U. The production of 2 zirs is carried out in both fast and thermal reactors.
From a weapons point of view, 2 ZZI is comparable to 2 39Pu: its radioactivity is 1/7 that of 2 39Pu (Ti/ 2 = 159200 liters versus 24100 liters for Pu), the critical mass of 2 zi is 60% higher than that of ^Pu (16 kg versus 10 kg), and the rate of spontaneous fission is 20 times higher (bth - ' versus 310 10). The neutron flux from 2 zzi is three times higher than that of 2 39Pi. Creating a nuclear charge based on 2 zi requires more effort than on ^Pi. The main obstacle is the presence of 232 U impurity in 2ZZI, the y-radiation of decay projects of which makes it difficult to work with 2ZZI and makes it easy to detect finished weapons. In addition, the short half-life of 2 3 2 U makes it an active source of alpha particles. 2 zi with 1% 232 and has three times stronger a-activity than weapons-grade plutonium and, accordingly, greater radiotoxicity. This a-activity causes the creation of neutrons in the light elements of the weapon charge. To minimize this problem, the presence of elements such as Be, B, F, Li should be minimal. The presence of a neutron background does not affect the operation of implosion systems, but cannon circuits require a high level of purity for light elements. The content of 23 2 U in weapons-grade 2 zis should not exceed 5 parts per million (0.0005%). In the fuel of thermal power reactors, the presence of 2 This is not harmful, and even desirable, because it reduces the possibility of using uranium for weapons purposes. After reprocessing the spent fuel and reusing the fuel, the 232U content reaches about 1 + 0.2%.
The decay of 2 zi occurs in the following directions:
A decay in 22 9Th (probability 10%, decay energy 4.909 MeV):
the energy of emitted yahr particles is 4.729 MeV (in 1.61% of cases), 4.784 MeV (in 13.2% of cases) and 4.824 MeV (in 84.4% of cases).
- - spontaneous division (probability
- - cluster decay with the formation of nuclide 28 Mg (decay probability less than 1.3*10_13%):
Cluster decay with the formation of the nuclide 24 Ne (decay probability 7.3-10-“%):
The decay chain of 2 zzi belongs to the neptunium series.
The specific radioactivity of 2 zi is 3.57-8 Bq/g, which corresponds to a-activity (and radiotoxicity) of -15% of plutonium. Just 1% 2 3 2 U increases radioactivity to 212 mCi/g.
Uran-234(Uranus II, UII) part of natural uranium (0.0055%), 2.445105 years, a-emitter (energy of a-particles 4.777 (72%) and
4.723 (28%) MeV), parent radionuclides: 2 h 8 Pu(a), 234 Pa(P), 234 Np(p +),
daughter isotope in 2 z”th. 
Typically, 234 U is in equilibrium with 2 h 8 u, decaying and forming at the same rate. Approximately half of the radioactivity of natural uranium is contributed by 234U. Typically, 234U is obtained by ion-exchange chromatography of old preparations of pure 2 × 8 Pu. During a-decay, *zRi yields 2 34U, so old preparations of 2 h 8 Ru are good sources of 2 34U. yuo g 238Pi contain after a year 776 mg 2 34U, after 3 years
2.2 g 2 34U. The concentration of 2 34U in highly enriched uranium is quite high due to preferential enrichment with light isotopes. Since 2 34u is a strong y-emitter, there are restrictions on its concentration in uranium intended for processing into fuel. Increased levels of 234i are acceptable for reactors, but reprocessed spent fuel already contains unacceptable levels of this isotope.
Decay of 234i occurs in the following directions:
A-decay at 2 3°Т (probability 100%, decay energy 4.857 MeV):
the energy of emitted alpha particles is 4.722 MeV (in 28.4% of cases) and 4.775 MeV (in 71.4% of cases).
- - spontaneous division (probability 1.73-10-9%).
- - cluster decay with the formation of nuclide 28 Mg (probability of decay 1.4-10%, according to other data 3.9-10%):
- - cluster decay with the formation of nuclides 2 4Ne and 26 Ne (decay probability 9-10", 2%, according to other data 2,3-10_11%):
The only known isomer is 2 34ti (Tx/ 2 = 33.5 μs).
The absorption cross section of 2 34U thermal neutrons is 100 barn, and for the resonance integral averaged over various intermediate neutrons it is 700 barn. Therefore, in thermal neutron reactors it is converted to fissile 235U at a faster rate than the much larger amount of 238U (with a cross-section of 2.7 barn) is converted to 2 39Ru. As a result, spent fuel contains less 2 34U than fresh fuel.
Uran-235 belongs to the 4P+3 family, capable of producing a fission chain reaction. This is the first isotope in which the reaction of forced nuclear fission under the influence of neutrons was discovered. By absorbing a neutron, 235U becomes 2 zbi, which is divided into two parts, releasing energy and emitting several neutrons. Fissile by neutrons of any energy and capable of spontaneous fission, the isotope 2 35U is part of natural ufan (0.72%), an a-emitter (energies 4.397 (57%) and 4.367 (18%) MeV), Ti/j=7.038-8 years, mother nuclides 2 35Pa, 2 35Np and 2 39Pu, daughter - 23Th. Spontaneous fission rate 2 3su 0.16 fission/s kg. When one 2 35U nucleus fissions, 200 MeV of energy = 3.210 p J is released, i.e. 18 TJ/mol=77 TJ/kg. The cross section of fission by thermal neutrons is 545 barns, and by fast neutrons - 1.22 barns, neutron yield: per fission act - 2.5, per absorbed neutron - 2.08.
Comment. The cross section for slow neutron capture to produce the isotope 2 sii (oo barn), so that the total slow neutron absorption cross section is 645 barn.

- - spontaneous fission (probability 7*10~9%);
- - cluster decay with the formation of nuclides 2 °Ne, 2 5Ne and 28 Mg (the probabilities, respectively, are 8-io_10%, 8-kg 10%, 8*10",0%):

Rice. 1.
The only known isomer is 2 35n»u (7/ 2 = 2b min).
Specific activity 2 35C 7.77-4 Bq/g. The critical mass of weapons-grade uranium (93.5% 2 35U) for a ball with a reflector is 15-7-23 kg.
2 » 5U fission is used in atomic weapons, for energy production and for the synthesis of important actinides. The chain reaction is maintained due to the excess of neutrons produced during the fission of 2 35C.
Uran-236 found naturally on Earth in trace quantities (there is more of it on the Moon), a-emitter (? 
Rice. 2. Radioactive family 4/7+2 (including -з 8 и).
In an atomic reactor, 2 sz absorbs a thermal neutron, after which it fissions with a probability of 82%, and with a probability of 18% it emits a y-quantum and turns into 2 sb and (for 100 fissioned nuclei 2 35U there are 22 formed nuclei 2 3 6 U) . In small quantities it is part of fresh fuel; accumulates when uranium is irradiated with neutrons in a reactor, and is therefore used as a “signaling device” for spent nuclear fuel. 2 h b and is formed as a by-product during the separation of isotopes by gas diffusion during the regeneration of used nuclear fuel. 236 U is a neutron poison formed in a power reactor; its presence in nuclear fuel is compensated for by a high level of enrichment 2 35 U.
2 z b and is used as a tracer of mixing ocean waters.
Uranium-237,T&= 6.75 days, beta and gamma emitter, can be obtained from nuclear reactions:

Detection 287 and is carried out along lines with Ey= o,ob MeV (36%), 0.114 MeV (0.06%), 0.165 MeV (2.0%), 0.208 MeV (23%)
237U is used in the radioactive tracer method in chemical research. Measuring the concentration (2-4°Am) in fallout from atomic weapons tests provides valuable information about the type of charge and the equipment used.
Uran-238- belongs to the 4P+2 family, fissile by high-energy neutrons (more than 1.1 MeV), capable of spontaneous fission, forms the basis of natural uranium (99.27%), a-emitter, 7’; /2=4>468-109 years, directly decays into 2 34Th, forms a number of genetically related radionuclides, and after 18 products turns into 206 Pb. Pure 2 3 8 U has a specific radioactivity of 1.22-104 Bq. The half-life is very long - about 10 16 years, so the probability of fission in relation to the main process - the emission of an alpha particle - is only 10" 7. One kilogram of uranium gives only 10 spontaneous fissions per second, and during the same time alpha particles emit 20 million nuclei. Mother nuclides: 2 4 2 Pu(a), *38ra(p-) 234Th, daughter T,/ 2 = 2 :i 4 Th.
Uranium-238 is formed as a result of the following decays:
2 (V0 4) 2 ] 8H 2 0. Among secondary minerals, hydrated calcium uranyl phosphate Ca(U0 2) 2 (P0 4) 2 -8H 2 0 is common. Often uranium in minerals is accompanied by other useful elements - titanium, tantalum, rare earths. Therefore, it is natural to strive for complex processing of uranium-containing ores.
Basic physical properties of uranium: atomic mass 238.0289 amu. (g/mol); atomic radius 138 pm (1 pm = 12 m); ionization energy (first electron 7.11 eV; electronic configuration -5f36d‘7s 2; oxidation states 6, 5, 4, 3; GP l = 113 2, 2 °; T t,1=3818°; density 19.05; specific heat capacity 0.115 JDKmol); tensile strength 450 MPa, heat of fusion 12.6 kJ/mol, heat of evaporation 417 kJ/mol, specific heat 0.115 J/(mol-K); molar volume 12.5 cm3/mol; characteristic Debye temperature © D =200K, temperature of transition to the superconducting state about.68K.
Uranium is a heavy, silvery-white, shiny metal. It is slightly softer than steel, malleable, flexible, has slight paramagnetic properties, and is pyrophoric in powder form. Uranium has three allotropic forms: alpha (orthorhombic, a-U, lattice parameters 0=285, b= 587, c=49b pm, stable up to 667.7°), beta (tetragonal, p-U, stable from 667.7 to 774.8°), gamma (with a cubic body-centered lattice, y-U, existing from 774.8° to melting points, frm=ii34 0), at which uranium is most malleable and convenient for processing.
At room temperature, the orthorhombic a-phase is stable; the prismatic structure consists of wavy atomic layers parallel to the plane ABC, in an extremely asymmetrical prismatic lattice. Within layers, atoms are tightly bonded, while the strength of bonds between atoms in adjacent layers is much weaker (Figure 4). This anisotropic structure makes it difficult to alloy uranium with other metals. Only molybdenum and niobium create solid-phase alloys with uranium. However, uranium metal can interact with many alloys, forming intermetallic compounds.
In the range 668^775° there is (3-uranium. The tetragonal type lattice has a layered structure with layers parallel to the plane ab in positions 1/4С, 1/2 With and 3/4C of the unit cell. At temperatures above 775°, y-uranium with a body-centered cubic lattice is formed. The addition of molybdenum allows the y-phase to be present at room temperature. Molybdenum forms a wide range of solid solutions with y-uranium and stabilizes the y-phase at room temperature. y-Uranium is much softer and more malleable than the brittle a- and (3-phases.
Neutron irradiation has a significant impact on the physical and mechanical properties of uranium, causing an increase in the size of the sample, a change in shape, as well as a sharp deterioration in the mechanical properties (creep, embrittlement) of uranium blocks during the operation of a nuclear reactor. The increase in volume is due to the accumulation in uranium during fission of impurities of elements with a lower density (translation 1% uranium into fragmentation elements increases the volume by 3.4%).

Rice. 4. Some crystal structures of uranium: a - a-uranium, b - p-uranium.
The most common methods for obtaining uranium in the metallic state are the reduction of their fluorides with alkali or alkaline earth metals or the electrolysis of molten salts. Uranium can also be obtained by metallothermal reduction from carbides with tungsten or tantalum.
The ability to easily give up electrons determines the reducing properties of uranium and its greater chemical activity. Uranium can interact with almost all elements except noble gases, acquiring oxidation states +2, +3, +4, +5, +6. In solution the main valence is 6+.
Rapidly oxidizing in air, metallic uranium is covered with an iridescent film of oxide. Fine uranium powder spontaneously ignites in air (at temperatures of 1504-175°), forming and;) Ov. At 1000°, uranium combines with nitrogen, forming yellow uranium nitride. Water can react with metal, slowly at low temperatures and quickly at high temperatures. Uranium reacts violently with boiling water and steam to release hydrogen, which forms a hydride with uranium
This reaction is more energetic than the combustion of uranium in oxygen. This chemical activity of uranium makes it necessary to protect uranium in nuclear reactors from contact with water.
Uranium dissolves in hydrochloric, nitric and other acids, forming U(IV) salts, but does not interact with alkalis. Uranium displaces hydrogen from inorganic acids and salt solutions of metals such as mercury, silver, copper, tin, platinum and gold. When shaken vigorously, the metal particles of uranium begin to glow.
The structural features of the electron shells of the uranium atom (the presence of ^/-electrons) and some of its physicochemical properties serve as the basis for classifying uranium as a member of the actinide series. However, there is a chemical analogy between uranium and Cr, Mo and W. Uranium is highly reactive and reacts with all elements except noble gases. In the solid phase, examples of U(VI) are uranyl trioxide U0 3 and uranyl chloride U0 2 C1 2. Uranium tetrachloride UC1 4 and uranium dioxide U0 2
Examples of U(IV). Substances containing U(IV) are usually unstable and become hexavalent when exposed to air for a long time.
Six oxides are installed in the uranium-oxygen system: UO, U0 2, U 4 0 9, and 3 Ov, U0 3. They are characterized by a wide range of homogeneity. U0 2 is a basic oxide, while U0 3 is amphoteric. U0 3 - interacts with water to form a number of hydrates, the most important of which are diuranic acid H 2 U 2 0 7 and uranic acid H 2 1U 4. With alkalis, U0 3 forms salts of these acids - uranates. When U0 3 is dissolved in acids, salts of the doubly charged uranyl cation U0 2 a+ are formed.
Uranium dioxide, U0 2, of stoichiometric composition is brown. As the oxygen content in the oxide increases, the color changes from dark brown to black. Crystal structure of the CaF 2 type, A = 0.547 nm; density 10.96 g/cm"* (the highest density among uranium oxides). T , pl =2875 0 , Tk „ = 3450°, D#°298 = -1084.5 kJ/mol. Uranium dioxide is a semiconductor with hole conductivity and a strong paramagnetic. MPC = o.015 mg/m3. Insoluble in water. At a temperature of -200° it adds oxygen, reaching the composition U0 2>25.
Uranium (IV) oxide can be prepared by the following reactions:
Uranium dioxide exhibits only basic properties; it corresponds to the basic hydroxide U(OH) 4, which is then converted into hydrated hydroxide U0 2 H 2 0. Uranium dioxide slowly dissolves in strong non-oxidizing acids in the absence of atmospheric oxygen with the formation of III + ions:
U0 2 + 2H 2 S0 4 ->U(S0 4) 2 + 2H 2 0. (38)
It is soluble in concentrated acids, and the rate of dissolution can be significantly increased by adding fluorine ion.
When dissolved in nitric acid, the formation of uranyl ion 1O 2 2+ occurs:
Triuranium octaoxide U 3 0s (uranium oxide) is a powder whose color varies from black to dark green; when strongly crushed, it turns olive-green in color. Large black crystals leave green streaks on the porcelain. Three crystal modifications of U 3 0 are known h: a-U 3 C>8 - rhombic crystal structure (space group C222; 0 = 0.671 nm; 6 = 1.197 nm; c = o.83 nm; d =0.839 nm); p-U 3 0e - rhombic crystal structure (space group Stst; 0=0.705 nm; 6=1.172 nm; 0=0.829 nm. The beginning of decomposition is oooo° (transitions to 100 2), MPC = 0.075 mg/m3.
U 3 C>8 can be obtained by the reaction:
By calcination U0 2, U0 2 (N0 3) 2, U0 2 C 2 0 4 3H 2 0, U0 4 -2H 2 0 or (NH 4) 2 U 2 0 7 at 750 0 in air or in an oxygen atmosphere (p = 150+750 mmHg) obtain stoichiometrically pure U 3 08.
When U 3 0s is calcined at T>oooo°, it is reduced to 10 0 2 , but upon cooling in air it returns to U 3 0s. U 3 0e dissolves only in concentrated strong acids. In hydrochloric and sulfuric acids a mixture of U(IV) and U(VI) is formed, and in nitric acid - uranyl nitrate. Dilute sulfuric and hydrochloric acids react very weakly with U 3 Os even when heated; the addition of oxidizing agents (nitric acid, pyrolusite) sharply increases the dissolution rate. Concentrated H 2 S0 4 dissolves U 3 Os to form U(S0 4) 2 and U0 2 S0 4 . Nitric acid dissolves U 3 Oe to form uranyl nitrate.
Uranium trioxide, U0 3 - a crystalline or amorphous substance of bright yellow color. Reacts with water. MAC = 0.075 mg/m3.
It is obtained by calcining ammonium polyuranates, uranium peroxide, uranyl oxalate at 300-500° and uranyl nitrate hexahydrate. This produces an orange powder of an amorphous structure with a density
6.8 g/cmz. The crystalline form of IU 3 can be obtained by oxidation of U 3 0 8 at temperatures of 450°h-750° in a flow of oxygen. There are six crystalline modifications of U0 3 (a, (3, y> §> ?, n) - U0 3 is hygroscopic and in moist air turns into uranyl hydroxide. Its heating at 520°-^6oo° gives a compound of composition 1U 2>9, further heating to 6oo° allows one to obtain U 3 Os.
Hydrogen, ammonia, carbon, alkali and alkaline earth metals reduce U0 3 to U0 2. When passing a mixture of gases HF and NH 3, UF 4 is formed. At higher valence, uranium exhibits amphoteric properties. When exposed to acids U0 3 or its hydrates, uranyl salts (U0 2 2+) are formed, colored yellow-green:
Most uranyl salts are highly soluble in water.
When fused with alkalis, U0 3 forms uranic acid salts - MDKH uranates:
With alkaline solutions, uranium trioxide forms salts of polyuranic acids - polyuranates DHM 2 0y1U 3 pH^O.
Uranic acid salts are practically insoluble in water.
The acidic properties of U(VI) are less pronounced than the basic ones.
Uranium reacts with fluorine at room temperature. The stability of higher halides decreases from fluorides to iodides. Fluorides UF 3, U4F17, U2F9 and UF 4 are non-volatile, and UFe is volatile. The most important fluorides are UF 4 and UFe.
Ftppippiyanir okgilya t"yanya ppptrkart according to the practice:
The reaction in a fluidized bed is carried out according to the equation:

It is possible to use fluorinating agents: BrF 3, CC1 3 F (Freon-11) or CC1 2 F 2 (Freon-12):
Uranium fluoride (1U) UF 4 (“green salt”) is a bluish-greenish to emerald-colored powder. G 11L = yuz6°; Гк,«,.=-1730°. DN° 29 8= 1856 kJ/mol. The crystal structure is monoclinic (sp. gp. C2/s; 0=1.273 nm; 5=1.075 nm; 0=0.843 nm; d= 6.7 nm; p=12b°20"; density 6.72 g/cm3. UF 4 is a stable, inactive, non-volatile compound, poorly soluble in water. The best solvent for UF 4 is fuming perchloric acid HC10 4. Dissolves in oxidizing acids to form a uranyl salt ; quickly dissolves in a hot solution of Al(N0 3) 3 or AlC1 3, as well as in a solution of boric acid acidified with H 2 S0 4, HC10 4 or HC1. Complexing agents that bind fluoride ions, for example, Fe3 +, Al3 +. or boric acid, also contribute to the dissolution of UF 4. With fluorides of other metals it forms a number of poorly soluble double salts (MeUFe, Me 2 UF6, Me 3 UF 7, etc. NH 4 UF 5 is of industrial importance).
U(IV) fluoride is an intermediate product in the preparation
both UF6 and uranium metal.
UF 4 can be obtained by reactions: 
or by electrolytic reduction of uranyl fluoride.
Uranium hexafluoride UFe - at room temperature, ivory-colored crystals with a high refractive index. Density
5.09 g/cmz, density of liquid UFe - 3.63 g/cmz. Volatile compound. Tvoag = 5^>5°> Gil=b4.5° (under pressure). The saturated vapor pressure reaches the atmosphere at 560°. Enthalpy of formation AH° 29 8 = -211b kJ/mol. The crystal structure is orthorhombic (space group. Rpt; 0=0.999 nm; fe= 0.8962 nm; c=o.5207 nm; d 5.060 nm (25 0). MPC - 0.015 mg/m3. From the solid state, UF6 can sublimate (sublimate) into a gas, bypassing the liquid phase over a wide range of pressures. Heat of sublimation at 50 0 50 kJ/mg. The molecule has no dipole moment, so UF6 does not associate. UFr vapor is an ideal gas.
It is obtained by the action of fluorine on its U compound:

In addition to gas-phase reactions, there are also liquid-phase reactions
producing UF6 using halofluorides, for example

There is a way to obtain UF6 without the use of fluorine - by oxidation of UF 4:
UFe does not react with dry air, oxygen, nitrogen and C0 2, but upon contact with water, even traces of it, it undergoes hydrolysis:
It interacts with most metals, forming their fluorides, which complicates the methods of its storage. Suitable vessel materials for working with UF6 are: when heated, Ni, Monel and Pt, in the cold - also Teflon, absolutely dry quartz and glass, copper and aluminum. At temperatures of 25-0°C it forms complex compounds with fluorides of alkali metals and silver of the type 3NaFUFr>, 3KF2UF6.
It dissolves well in various organic liquids, inorganic acids and all halofluorides. Inert to dry 0 2, N 2, C0 2, C1 2, Br 2. UFr is characterized by reduction reactions with most pure metals. UF6 reacts vigorously with hydrocarbons and other organic substances, so closed containers with UFe can explode. UF6 in the range of 25 -r100° forms complex salts with fluorides of alkali and other metals. This property is used in technology for selective extraction of UF
Uranium hydrides UH 2 and UH 3 occupy an intermediate position between salt-like hydrides and hydrides of the type of solid solutions of hydrogen in the metal.
When uranium reacts with nitrogen, nitrides are formed. There are four known phases in the U-N system: UN (uranium nitride), a-U 2 N 3 (sesquinitride), p- U 2 N 3 and UN If90. It is not possible to achieve the composition UN 2 (dinitride). Syntheses of uranium mononitride UN are reliable and well controlled, which are best carried out directly from the elements. Uranium nitrides are powdery substances, the color of which varies from dark gray to gray; look like metal. UN has a cubic face-centered crystal structure, like NaCl (0 = 4.8892 A); (/=14.324, 7^=2855°, stable in vacuum up to 1700 0. It is prepared by reacting U or U hydride with N 2 or NH 3 , decomposition of higher U nitrides at 1300° or their reduction with uranium metal. U 2 N 3 is known in two polymorphic modifications: cubic a and hexagonal p (0 = 0.3688 nm, 6 = 0.5839 nm), releases N 2 in a vacuum above 8oo°. It is obtained by reducing UN 2 with hydrogen. UN2 dinitride is synthesized by reacting U with N2 under high N2 pressure. Uranium nitrides are easily soluble in acids and alkali solutions, but are decomposed by molten alkalis.
Uranium nitride is obtained by two-stage carbothermic reduction of uranium oxide:
Heating in argon at 7M450 0 for 10*20 hours
Uranium nitride of a composition close to dinitride, UN 2, can be obtained by exposing UF 4 to ammonia at high temperature and pressure.
Uranium dinitride decomposes when heated:
Uranium nitride, enriched at 2 35 U, has a higher fission density, thermal conductivity and melting point than uranium oxides - the traditional fuel of modern power reactors. It also has good mechanical properties and stability superior to traditional fuels. Therefore, this compound is considered as a promising basis for nuclear fuel in fast neutron reactors (generation IV nuclear reactors).
Comment. It is very useful to enrich UN by ‘5N, because .4 N tends to capture neutrons, generating the radioactive isotope 14 C through the (n,p) reaction.
Uranium carbide UC 2 (?-phase) is a light gray crystalline substance with a metallic luster. In the U-C system (uranium carbides), there are UC 2 (?-phase), UC 2 (b 2-phase), U 2 C 3 (e-phase), UC (b 2-phase) - uranium carbides. Uranium dicarbide UC 2 can be obtained by the reactions:
U + 2C^UC 2 (54v)
Uranium carbides are used as fuel for nuclear reactors; they are promising as fuel for space rocket engines.
Uranyl nitrate, uranyl nitrate, U0 2 (N0 3) 2 -6H 2 0. The role of the metal in this salt is played by the uranyl 2+ cation. Yellow crystals with a greenish tint, easily soluble in water. An aqueous solution is acidic. Soluble in ethanol, acetone and ether, insoluble in benzene, toluene and chloroform. When heated, the crystals melt and release HN0 3 and H 2 0. Crystalline hydrate is easily evaporated in air. A characteristic reaction is that under the action of NH 3 a yellow precipitate of ammonium uranium is formed.
Uranium is capable of forming metal-organic compounds. Examples are cyclopentadienyl derivatives of the composition U(C 5 H 5) 4 and their halogen-substituted u(C 5 H 5) 3 G or u(C 5 H 5) 2 G 2.
In aqueous solutions, uranium is most stable in the oxidation state of U(VI) in the form of the uranyl ion U0 2 2+. To a lesser extent, it is characterized by the U(IV) state, but it can even occur in the U(III) form. The oxidation state of U(V) can exist as the IO2+ ion, but this state is rarely observed due to its tendency to disproportionation and hydrolysis.
In neutral and acidic solutions, U(VI) exists in the form of U0 2 2+ - a yellow uranyl ion. Well-soluble uranyl salts include nitrate U0 2 (N0 3) 2, sulfate U0 2 S0 4, chloride U0 2 C1 2, fluoride U0 2 F 2, acetate U0 2 (CH 3 C00) 2. These salts are released from solutions in the form of crystalline hydrates with different numbers of water molecules. Slightly soluble uranyl salts are: oxalate U0 2 C 2 0 4, phosphates U0 2 HP0., and UO2P2O4, ammonium uranyl phosphate UO2NH4PO4, sodium uranyl vanadate NaU0 2 V0 4, ferrocyanide (U0 2) 2. The uranyl ion is characterized by a tendency to form complex compounds. Thus, complexes with fluorine ions of the -, 4- type are known; nitrate complexes ‘ and 2*; sulfuric acid complexes 2 " and 4-; carbonate complexes 4 " and 2 ", etc. When alkalis act on solutions of uranyl salts, sparingly soluble precipitates of diuranates of the type Me 2 U 2 0 7 are released (monouranates Me 2 U0 4 are not isolated from solutions, they are obtained by fusion uranium oxides with alkalis). Polyuranates Me 2 U n 0 3 n+i are known (for example, Na 2 U60i 9).
U(VI) is reduced in acidic solutions to U(IV) by iron, zinc, aluminum, sodium hydrosulfite, and sodium amalgam. The solutions are colored green. Alkalis precipitate from them hydroxide U0 2 (0H) 2, hydrofluoric acid - fluoride UF 4 -2.5H 2 0, oxalic acid - oxalate U(C 2 0 4) 2 -6H 2 0. The U 4+ ion has a tendency to form complexes less than that of uranyl ions.
Uranium (IV) in solution is in the form of U 4+ ions, which are highly hydrolyzed and hydrated:
In acidic solutions, hydrolysis is suppressed.
Uranium (VI) in solution forms the uranyl oxocation - U0 2 2+ Numerous uranyl compounds are known, examples of which are: U0 3, U0 2 (C 2 H 3 0 2) 2, U0 2 C0 3 -2(NH 4) 2 C0 3 U0 2 C0 3, U0 2 C1 2, U0 2 (0H) 2, U0 2 (N0 3) 2, UO0SO4, ZnU0 2 (CH 3 C00) 4, etc.
Upon hydrolysis of uranyl ion, a number of multinuclear complexes are formed:
With further hydrolysis, U 3 0s(0H) 2 and then U 3 0 8 (0H) 4 2 - appear.
For the qualitative detection of uranium, methods of chemical, luminescent, radiometric and spectral analyzes are used. Chemical methods are predominantly based on the formation of colored compounds (for example, red-brown color of a compound with ferrocyanide, yellow with hydrogen peroxide, blue with arsenazo reagent). The luminescent method is based on the ability of many uranium compounds to produce a yellowish-greenish glow when exposed to UV rays.
Quantitative determination of uranium is carried out by various methods. The most important of them are: volumetric methods, consisting of the reduction of U(VI) to U(IV) followed by titration with solutions of oxidizing agents; gravimetric methods - precipitation of uranates, peroxide, U(IV) cupferranates, hydroxyquinolate, oxalate, etc. followed by calcination at 00° and weighing U 3 0s; polarographic methods in a nitrate solution make it possible to determine 10*7-g10-9 g of uranium; numerous colorimetric methods (for example, with H 2 0 2 in an alkaline medium, with the arsenazo reagent in the presence of EDTA, with dibenzoylmethane, in the form of a thiocyanate complex, etc.); luminescent method, which makes it possible to determine when fused with NaF to Yu 11 g uranium.
235U belongs to radiation hazard group A, the minimum significant activity is MZA = 3.7-10 4 Bq, 2 3 8 and - to group D, MZA = 3.7-6 Bq (300 g).
URANUS (named after the planet Uranus discovered shortly before; lat. uranium * a. uranium; n. Uran; f. uranium; i. uranio), U, is a radioactive chemical element of group III of the Mendeleev periodic system, atomic number 92, atomic mass 238.0289, belongs to actinides. Natural uranium consists of a mixture of three isotopes: 238 U (99.282%, T 1/2 4,468.10 9 years), 235 U (0.712%, T 1/2 0.704.10 9 years), 234 U (0.006%, T 1/2 0.244.10 6 years). There are also 11 known artificial radioactive isotopes of uranium with mass numbers from 227 to 240. 238 U and 235 U are the founders of two natural decay series, as a result of which they turn into stable isotopes 206 Pb and 207 Pb, respectively.
Uranium was discovered in 1789 in the form of UO 2 by the German chemist M. G. Klaproth. Uranium metal was obtained in 1841 by the French chemist E. Peligot. For a long time, uranium had very limited use, and only with the discovery of radioactivity in 1896 did its study and use begin.
Properties of uranium
In its free state, uranium is a light gray metal; below 667.7°C it is characterized by an orthorhombic (a=0.28538 nm, b=0.58662 nm, c=0.49557 nm) crystal lattice (a-modification), in the temperature range 667.7-774°C - tetragonal (a = 1.0759 nm, c = 0.5656 nm; G-modification), at a higher temperature - body-centered cubic lattice (a = 0.3538 nm, g-modification). Density 18700 kg/m 3, melting point 1135°C, boiling point about 3818°C, molar heat capacity 27.66 J/(mol.K), electrical resistivity 29.0.10 -4 (Ohm.m), thermal conductivity 22, 5 W/(m.K), temperature coefficient of linear expansion 10.7.10 -6 K -1. The temperature of transition of uranium to the superconducting state is 0.68 K; weak paramagnetic, specific magnetic susceptibility 1.72.10 -6. The nuclei 235 U and 233 U fission spontaneously, as well as upon the capture of slow and fast neutrons, 238 U fission only upon the capture of fast (more than 1 MeV) neutrons. When slow neutrons are captured, 238 U turns into 239 Pu. The critical mass of uranium (93.5% 235U) in aqueous solutions is less than 1 kg, for an open ball it is about 50 kg; for 233 U critical mass is approximately 1/3 of the critical mass of 235 U.
Education and keeping in nature
The main consumer of uranium is nuclear energy (nuclear reactors, nuclear power plants). In addition, uranium is used to produce nuclear weapons. All other areas of uranium use are of strictly subordinate importance.
Contents of the article
URANUS, U (uranium), a metal chemical element of the actinide family, which includes Ac, Th, Pa, U and transuranium elements (Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr). Uranium has gained prominence due to its use in nuclear weapons and nuclear power. Uranium oxides are also used to color glass and ceramics.
Being in nature.
The uranium content in the earth's crust is 0.003%, and it is found in the surface layer of the earth in the form of four types of sediments. Firstly, these are veins of uraninite, or uranium pitch (uranium dioxide UO 2), very rich in uranium, but rare. They are accompanied by radium deposits, since radium is a direct product of the isotope decay of uranium. Such veins are found in Zaire, Canada (Great Bear Lake), Czech Republic and France. The second source of uranium is conglomerates of thorium and uranium ores together with ores of other important minerals. Conglomerates usually contain sufficient amounts of gold and silver to be recovered, with uranium and thorium being associated elements. Large deposits of these ores are located in Canada, South Africa, Russia and Australia. The third source of uranium is sedimentary rocks and sandstones rich in the mineral carnotite (potassium uranyl vanadate), which contains, in addition to uranium, a significant amount of vanadium and other elements. Such ores are found in the western states of the United States. Iron-uranium shales and phosphate ores constitute a fourth source of sediment. Rich deposits are found in the shales of Sweden. Some phosphate ores in Morocco and the United States contain significant amounts of uranium, and phosphate deposits in Angola and the Central African Republic are even richer in uranium. Most lignites and some coals usually contain uranium impurities. Uranium-rich lignite deposits have been found in North and South Dakota (USA) and bituminous coals in Spain and the Czech Republic.
Opening.
Uranus was discovered in 1789 by the German chemist M. Klaproth, who named the element in honor of the discovery of the planet Uranus 8 years earlier. (Klaproth was the leading chemist of his time; he also discovered other elements, including Ce, Ti and Zr.) In fact, the substance Klaproth obtained was not elemental uranium, but an oxidized form of it, and elemental uranium was first obtained by the French chemist E. .Peligo in 1841. From the moment of discovery until the 20th century. uranium did not have the significance it has today, although many of its physical properties, as well as its atomic mass and density, were determined. In 1896, A. Becquerel established that uranium salts have radiation that illuminates a photographic plate in the dark. This discovery activated chemists to research in the field of radioactivity and in 1898, the French physicists spouses P. Curie and M. Sklodowska-Curie isolated salts of the radioactive elements polonium and radium, and E. Rutherford, F. Soddy, K. Fayans and other scientists developed the theory of radioactive decay, which laid the foundations of modern nuclear chemistry and nuclear energy.
First uses of uranium.
Although the radioactivity of uranium salts was known, its ores in the first third of this century were used only to obtain accompanying radium, and uranium was considered an undesirable by-product. Its use was concentrated mainly in ceramic technology and metallurgy; Uranium oxides were widely used to color glass in colors ranging from pale yellow to dark green, which contributed to the development of inexpensive glass production. Today, products from these industries are identified as fluorescent under ultraviolet rays. During World War I and shortly thereafter, uranium in the form of carbide was used in the production of tool steels, similar to Mo and W; 4–8% uranium replaced tungsten, the production of which was limited at the time. To obtain tool steels in 1914–1926, several tons of ferrouranium containing up to 30% (mass) U were produced annually. However, this use of uranium did not last long.
Modern uses of uranium.
The uranium industry began to take shape in 1939, when the fission of the uranium isotope 235 U was carried out, which led to the technical implementation of controlled chain reactions of uranium fission in December 1942. This was the birth of the age of the atom, when uranium grew from an insignificant element to one of the most important elements in life society. The military importance of uranium for the production of the atomic bomb and its use as fuel in nuclear reactors caused the demand for uranium to increase astronomically. The chronology of the growth in uranium demand based on the history of sediments in Great Bear Lake (Canada) is interesting. In 1930, tar blende, a mixture of uranium oxides, was discovered in this lake, and in 1932, radium purification technology was established in this area. From each ton of ore (resin blende) 1 g of radium and about half a ton of by-product, uranium concentrate, were obtained. However, there was little radium and its mining was stopped. From 1940 to 1942, development was resumed and uranium ore began to be shipped to the United States. In 1949, similar uranium purification, with some improvements, was used to produce pure UO 2 . This production has grown and is now one of the largest uranium production facilities.
Properties.
Uranium is one of the heaviest elements found in nature. Pure metal is very dense, ductile, electropositive with low electrical conductivity, and highly reactive.
Uranium has three allotropic modifications: a-uranium (orthorhombic crystal lattice), exists in the range from room temperature to 668 ° C; b-uranium (complex crystal lattice of tetragonal type), stable in the range of 668–774° C; g-uranium (body-centered cubic crystal lattice), stable from 774°C up to the melting point (1132°C). Since all isotopes of uranium are unstable, all its compounds exhibit radioactivity.
Isotopes of uranium
238 U, 235 U, 234 U occur in nature in a ratio of 99.3:0.7:0.0058, and 236 U occurs in trace amounts. All other isotopes of uranium from 226 U to 242 U are obtained artificially. The isotope 235 U is particularly important. Under the influence of slow (thermal) neutrons, it divides, releasing enormous energy. Complete fission of 235 U leads to the release of a “thermal energy equivalent” of 2H 10 7 kWh h/kg. The fission of 235 U can be used not only to produce large amounts of energy, but also to synthesize other important actinide elements. Natural isotope uranium can be used in nuclear reactors to produce neutrons produced by the fission of 235 U, while excess neutrons not required by the chain reaction can be captured by another natural isotope, resulting in the production of plutonium:
When 238 U is bombarded with fast neutrons, the following reactions occur:
According to this scheme, the most common isotope 238 U can be converted into plutonium-239, which, like 235 U, is also capable of fission under the influence of slow neutrons.
Currently, a large number of artificial isotopes of uranium have been obtained. Among them, 233 U is particularly notable because it also fissions when interacting with slow neutrons.
Some other artificial isotopes of uranium are often used as radioactive tracers in chemical and physical research; this is first of all b- emitter 237 U and a- emitter 232 U.
Connections.
Uranium, a highly reactive metal, has oxidation states from +3 to +6, is close to beryllium in the activity series, interacts with all non-metals and forms intermetallic compounds with Al, Be, Bi, Co, Cu, Fe, Hg, Mg, Ni, Pb, Sn and Zn. Finely crushed uranium is especially reactive and at temperatures above 500 ° C it often enters into reactions characteristic of uranium hydride. Lump uranium or shavings burn brightly at 700–1000° C, and uranium vapor burns already at 150–250° C; uranium reacts with HF at 200–400° C, forming UF 4 and H 2 . Uranium dissolves slowly in concentrated HF or H 2 SO 4 and 85% H 3 PO 4 even at 90 ° C, but easily reacts with conc. HCl and less active with HBr or HI. The most active and rapid reactions of uranium with dilute and concentrated HNO 3 occur with the formation of uranyl nitrate ( see below). In the presence of HCl, uranium quickly dissolves in organic acids, forming organic U4+ salts. Depending on the degree of oxidation, uranium forms several types of salts (the most important among them are with U 4+, one of them UCl 4 is an easily oxidized green salt); uranyl salts (radical UO 2 2+) of the type UO 2 (NO 3) 2 are yellow in color and fluoresce green. Uranyl salts are formed by dissolving the amphoteric oxide UO 3 (yellow color) in an acidic medium. In an alkaline environment, UO 3 forms uranates such as Na 2 UO 4 or Na 2 U 2 O 7. The latter compound (“yellow uranyl”) is used for the manufacture of porcelain glazes and in the production of fluorescent glasses.
Uranium halides were widely studied in 1940–1950, as they were used to develop methods for separating uranium isotopes for the atomic bomb or nuclear reactor. Uranium trifluoride UF 3 was obtained by the reduction of UF 4 with hydrogen, and uranium tetrafluoride UF 4 is obtained in various ways by reactions of HF with oxides such as UO 3 or U 3 O 8 or by electrolytic reduction of uranyl compounds. Uranium hexafluoride UF 6 is obtained by fluorination of U or UF 4 with elemental fluorine or by the action of oxygen on UF 4 . Hexafluoride forms transparent crystals with a high refractive index at 64 ° C (1137 mm Hg); the compound is volatile (under normal pressure it sublimes at 56.54 ° C). Uranium oxohalides, for example, oxofluorides, have the composition UO 2 F 2 (uranyl fluoride), UOF 2 (uranium oxide difluoride).
In recent years, the topic of nuclear energy has become increasingly relevant. To produce nuclear energy, it is common to use a material such as uranium. It is a chemical element belonging to the actinide family.
The chemical activity of this element determines the fact that it is not contained in free form. For its production, mineral formations called uranium ores are used. They concentrate such an amount of fuel that allows the extraction of this chemical element to be considered economically rational and profitable. At the moment, in the bowels of our planet the content of this metal exceeds the reserves of gold in 1000 times(cm. ). In general, deposits of this chemical element in soil, aquatic environment and rock are estimated at more than 5 million tons.
In the free state, uranium is a gray-white metal, which is characterized by 3 allotropic modifications: rhombic crystalline, tetragonal and body-centered cubic lattices. The boiling point of this chemical element is 4200 °C.
Uranium is a chemically active material. In air, this element slowly oxidizes, easily dissolves in acids, reacts with water, but does not interact with alkalis.

Uranium ores in Russia are usually classified according to various criteria. Most often they differ in terms of education. Yes, there are endogenous, exogenous and metamorphogenic ores. In the first case, they are mineral formations formed under the influence of high temperatures, humidity and pegmatite melts. Exogenous uranium mineral formations occur in surface conditions. They can form directly on the surface of the earth. This occurs due to the circulation of groundwater and the accumulation of sediments. Metamorphogenic mineral formations appear as a result of the redistribution of initially dispersed uranium.
According to the level of uranium content, these natural formations can be:
- super rich (over 0.3%);
- rich (from 0.1 to 0.3%);
- privates (from 0.05 to 0.1%);
- poor (from 0.03 to 0.05%);
- off-balance sheet (from 0.01 to 0.03%).
Modern uses of uranium

Today, uranium is most often used as fuel for rocket engines and nuclear reactors. Given the properties of this material, it is also intended to increase the power of a nuclear weapon. This chemical element has also found its use in painting. It is actively used as yellow, green, brown and black pigments. Uranium is also used to make cores for armor-piercing projectiles.
Mining uranium ore in Russia: what is needed for this?

The extraction of radioactive ores is carried out using three main technologies. If ore deposits are concentrated as close as possible to the surface of the earth, then it is customary to use open-pit technology for their extraction. It involves the use of bulldozers and excavators, which dig large holes and load the resulting minerals into dump trucks. Then it is sent to the processing complex.
When this mineral formation is located deeply, it is customary to use underground mining technology, which involves creating a mine up to 2 kilometers deep. The third technology differs significantly from the previous ones. In-ground leaching to develop uranium deposits involves drilling wells through which sulfuric acid is pumped into the deposits. Next, another well is drilled, which is necessary to pump the resulting solution to the surface of the earth. Then it goes through a sorption process, which allows the salts of this metal to be collected on a special resin. The last stage of SPV technology is cyclic treatment of the resin with sulfuric acid. Thanks to this technology, the concentration of this metal becomes maximum.
Uranium ore deposits in Russia
Russia is considered one of the world leaders in the mining of uranium ores. Over the past few decades, Russia has consistently ranked among the top 7 leading countries in this indicator.
The largest deposits of these natural mineral formations are:
The largest uranium mining deposits in the world - leading countries

Australia is considered the world leader in uranium mining. More than 30% of all world reserves are concentrated in this state. The largest Australian deposits are Olympic Dam, Beverly, Ranger and Honemoon.
Australia's main competitor is Kazakhstan, which contains almost 12% of the world's fuel reserves. Canada and South Africa each contain 11% of the world's uranium reserves, Namibia - 8%, Brazil - 7%. Russia closes the top seven with 5%. The list of leaders also includes countries such as Namibia, Ukraine and China.
The world's largest uranium deposits are:
| Field | Country | Start processing |
| Olympic Dam | Australia | 1988 |
| Rossing | Namibia | 1976 |
| McArthur River | Canada | 1999 |
| Inkai | Kazakhstan | 2007 |
| Dominion | South Africa | 2007 |
| Ranger | Australia | 1980 |
| Kharasan | Kazakhstan | 2008 |
Reserves and production volumes of uranium ore in Russia
The explored reserves of uranium in our country are estimated at more than 400 thousand tons. At the same time, the predicted resources are more than 830 thousand tons. As of 2017, there are 16 uranium deposits in Russia. Moreover, 15 of them are concentrated in Transbaikalia. The main deposit of uranium ore is considered to be the Streltsovskoe ore field. In most domestic deposits, production is carried out using the shaft method.
- Uranium was discovered back in the 18th century. In 1789, the German scientist Martin Klaproth managed to produce metal-like uranium from ore. Interestingly, this scientist is also the discoverer of titanium and zirconium.
- Uranium compounds are actively used in the field of photography. This element is used to color positives and enhance negatives.
- The main difference between uranium and other chemical elements is its natural radioactivity. Uranium atoms tend to change independently over time. At the same time, they emit rays invisible to the human eye. These rays are divided into 3 types - gamma, beta and alpha radiation (see).
; atomic number 92, atomic mass 238.029; metal. Natural Uranium consists of a mixture of three isotopes: 238 U - 99.2739% with a half-life T ½ = 4.51 10 9 years, 235 U - 0.7024% (T ½ = 7.13 10 8 years) and 234 U - 0.0057% (T ½ = 2.48·10 5 years).
Of the 11 artificial radioactive isotopes with mass numbers from 227 to 240, the long-lived one is 233 U (T ½ = 1.62·10 5 years); it is obtained by neutron irradiation of thorium. 238 U and 235 U are the ancestors of two radioactive series.
Historical information. Uranium was discovered in 1789 by the German chemist M. G. Klaproth and named by him in honor of the planet Uranus, discovered by W. Herschel in 1781. In the metallic state, Uranium was obtained in 1841 by the French chemist E. Peligo during the reduction of UCl 4 with potassium metal. Initially, Uranus was assigned an atomic mass of 120, and only in 1871 D.I. Mendeleev came to the conclusion that this value should be doubled.
For a long time, uranium was of interest only to a narrow circle of chemists and found limited use in the production of paints and glass. With the discovery of the phenomenon of radioactivity in uranium in 1896 and radium in 1898, industrial processing of uranium ores began in order to extract and use radium in scientific research and medicine. Since 1942, after the discovery of nuclear fission in 1939, uranium has become the main nuclear fuel.
Distribution of Uranus in nature. Uranium is a characteristic element for the granite layer and sedimentary shell of the earth's crust. The average content of Uranium in the earth's crust (clarke) is 2.5 10 -4% by mass, in acidic igneous rocks 3.5 10 -4%, in clays and shales 3.2 10 -4%, in basic rocks 5 ·10 -5%, in ultrabasic rocks of the mantle 3·10 -7%. Uranium migrates vigorously in cold and hot, neutral and alkaline waters in the form of simple and complex ions, especially in the form of carbonate complexes. Redox reactions play an important role in the geochemistry of Uranium, since Uranium compounds, as a rule, are highly soluble in waters with an oxidizing environment and poorly soluble in waters with a reducing environment (for example, hydrogen sulfide).
About 100 Uranium minerals are known; 12 of them are of industrial importance. Over the course of geological history, the content of Uranium in the earth's crust has decreased due to radioactive decay; This process is associated with the accumulation of Pb and He atoms in the earth's crust. The radioactive decay of Uranium plays an important role in the energy of the earth's crust, being a significant source of deep heat.
Physical properties of Uranium. Uranium is similar in color to steel and is easy to process. It has three allotropic modifications - α, β and γ with phase transformation temperatures: α → β 668.8 °C, β → γ 772.2 °C; The α-form has an orthorhombic lattice (a = 2.8538Å, b = 5.8662Å, c = 4.9557Å), the β-form has a tetragonal lattice (at 720 °C a = 10.759Å, b = 5.656Å), the γ-form - body-centered cubic lattice (at 850 °C a = 3.538 Å). The density of Uranium in α-form (25 °C) is 19.05 g/cm 3 ; t pl 1132 °C; boiling point 3818 °C; thermal conductivity (100-200 °C), 28.05 W/(m K), (200-400 °C) 29.72 W/(m K); specific heat capacity (25 °C) 27.67 kJ/(kg K); specific electrical resistivity at room temperature is about 3·10 -7 ohm·cm, at 600 °C 5.5·10 -7 ohm·cm; has superconductivity at 0.68 K; weak paramagnetic, specific magnetic susceptibility at room temperature 1.72·10 -6.
The mechanical properties of Uranium depend on its purity and on the modes of mechanical and thermal treatment. The average value of the elastic modulus for cast Uranium is 20.5·10 -2 Mn/m 2 ; tensile strength at room temperature 372-470 Mn/m2; strength increases after hardening from β- and γ-phases; average Brinell hardness 19.6-21.6·10 2 Mn/m 2 .
Irradiation by a neutron flow (which takes place in a nuclear reactor) changes the physical and mechanical properties of Uranium: creep develops and fragility increases, deformation of products is observed, which forces the use of Uranium in nuclear reactors in the form of various uranium alloys.
Uranium is a radioactive element. The nuclei 235 U and 233 U fission spontaneously, as well as during the capture of both slow (thermal) and fast neutrons with an effective fission cross section of 508 10 -24 cm 2 (508 barn) and 533 10 -24 cm 2 (533 barn) respectively. 238 U nuclei fission upon capturing only fast neutrons with an energy of at least 1 MeV; when capturing slow neutrons, 238 U turns into 239 Pu, the nuclear properties of which are close to 235 U. The critical mass of Uranium (93.5% 235 U) in aqueous solutions is less than 1 kg, for an open ball - about 50 kg, for a ball with a reflector - 15-23 kg; critical mass 233 U is approximately 1/3 of the critical mass 235 U.
Chemical properties of Uranium. The configuration of the outer electron shell of the Uranium atom is 7s 2 6d l 5f 3. Uranium is a reactive metal; in compounds it exhibits oxidation states of +3, +4, + 5, +6, sometimes +2; the most stable compounds are U (IV) and U (VI). In air it slowly oxidizes with the formation of an oxide (IV) film on the surface, which does not protect the metal from further oxidation. In its powdered state, Uranium is pyrophoric and burns with a bright flame. With oxygen it forms oxide (IV) UO 2, oxide (VI) UO 3 and a large number of intermediate oxides, the most important of which is U 3 O 8. These intermediate oxides have properties similar to UO 2 and UO 3 . At high temperatures, UO 2 has a wide range of homogeneity from UO 1.60 to UO 2.27. With fluorine at 500-600 ° C it forms UF 4 tetrafluoride (green needle-shaped crystals, slightly soluble in water and acids) and UF 6 hexafluoride (a white crystalline substance that sublimes without melting at 56.4 ° C); with sulfur - a number of compounds, of which US (nuclear fuel) is the most important. When Uranium reacts with hydrogen at 220 °C, the hydride UH 3 is obtained; with nitrogen at temperatures from 450 to 700 ° C and atmospheric pressure - U 4 N 7 nitride; at a higher nitrogen pressure and the same temperature, UN, U 2 N 3 and UN 2 can be obtained; with carbon at 750-800 °C - monocarbide UC, dicarbide UC 2, as well as U 2 C 3; with metals it forms alloys of various types. Uranium reacts slowly with boiling water to form UO 2 nH 2, with water vapor - in the temperature range 150-250 ° C; soluble in hydrochloric and nitric acids, slightly soluble in concentrated hydrofluoric acid. U(VI) is characterized by the formation of the uranyl ion UO 2 2+; uranyl salts are yellow in color and are highly soluble in water and mineral acids; U(IV) salts are green and less soluble; uranyl ion is extremely capable of complex formation in aqueous solutions with both inorganic and organic substances; The most important for technology are carbonate, sulfate, fluoride, phosphate and other complexes. A large number of uranates (salts of uranic acid not isolated in pure form) are known, the composition of which varies depending on the conditions of production; All uranates have low solubility in water.
Uranium and its compounds are radiation and chemically toxic. The maximum permissible dose (MAD) for occupational exposure is 5 rem per year.
Receiving Uranus. Uranium is obtained from uranium ores containing 0.05-0.5% U. The ores are practically not enriched, with the exception of a limited radiometric sorting method based on the γ-radiation of radium, which always accompanies uranium. Basically, ores are leached with solutions of sulfuric, sometimes nitric acids or soda solutions with the transfer of Uranium into an acidic solution in the form of UO 2 SO 4 or complex anions 4-, and into a soda solution - in the form of 4-. To extract and concentrate Uranium from solutions and pulps, as well as to purify it from impurities, sorption on ion exchange resins and extraction with organic solvents (tributyl phosphate, alkylphosphoric acids, amines) are used. Next, ammonium or sodium uranates or U(OH) 4 hydroxide are precipitated from the solutions by adding alkali. To obtain compounds of high purity, technical products are dissolved in nitric acid and subjected to refining purification operations, the final products of which are UO 3 or U 3 O 8; these oxides are reduced at 650-800 °C by hydrogen or dissociated ammonia to UO 2, followed by its conversion to UF 4 by treatment with hydrogen fluoride gas at 500-600 °C. UF 4 can also be obtained by precipitation of crystalline hydrate UF 4 nH 2 O with hydrofluoric acid from solutions, followed by dehydration of the product at 450 °C in a stream of hydrogen. In industry, the main method of obtaining Uranium from UF 4 is its calcium-thermal or magnesium-thermal reduction with the release of Uranium in the form of ingots weighing up to 1.5 tons. The ingots are refined in vacuum furnaces.
A very important process in Uranium technology is the enrichment of its 235 U isotope above the natural content in ores or the isolation of this isotope in its pure form, since 235 U is the main nuclear fuel; This is done by gas thermal diffusion, centrifugal and other methods based on the difference in the masses of 238 U and 235 U; in separation processes, uranium is used in the form of volatile hexafluoride UF 6. When obtaining highly enriched Uranium or isotopes, their critical masses are taken into account; the most convenient method in this case is the reduction of uranium oxides with calcium; the resulting CaO slag is easily separated from Uranium by dissolution in acids. To obtain powdered uranium, oxide (IV), carbides, nitrides and other refractory compounds, powder metallurgy methods are used.
Application of Uranus. Uranium metal or its compounds are used primarily as nuclear fuel in nuclear reactors. A natural or low-enriched mixture of Uranium isotopes is used in stationary reactors of nuclear power plants, a highly enriched product is used in nuclear power plants or in reactors operating on fast neutrons. 235 U is the source of nuclear energy in nuclear weapons. 238 U serves as a source of secondary nuclear fuel - plutonium.
Uranium in the body. It is found in microquantities (10 -5 -10 -8%) in the tissues of plants, animals and humans. In plant ash (with a Uranium content of about 10 -4% in the soil), its concentration is 1.5·10 -5%. To the greatest extent, Uranium is accumulated by some fungi and algae (the latter actively participate in the biogenic migration of Uranium along the chain water - aquatic plants - fish - humans). Uranium enters the body of animals and humans with food and water in the gastrointestinal tract, with air in the respiratory tract, as well as through the skin and mucous membranes. Uranium compounds are absorbed in the gastrointestinal tract - about 1% of the incoming amount of soluble compounds and no more than 0.1% of sparingly soluble ones; 50% and 20% are absorbed in the lungs, respectively. Uranium is distributed unevenly in the body. The main depot (places of deposition and accumulation) is the spleen, kidneys, skeleton, liver and, when inhaling poorly soluble compounds, the lungs and bronchopulmonary lymph nodes. Uranium (in the form of carbonates and complexes with proteins) does not circulate in the blood for a long time. The content of uranium in the organs and tissues of animals and humans does not exceed 10 -7 g/g. Thus, cattle blood contains 1·10 -8 g/ml, liver 8·10 -8 g/g, muscles 4·10 -11 g/g, spleen 9·10 8-8 g/g. The content of Uranium in human organs is: in the liver 6·10 -9 g/g, in the lungs 6·10 -9 -9·10 -9 g/g, in the spleen 4.7·10 -7 g/g, in the blood 4-10 -10 g/ml, in the kidneys 5.3·10 -9 (cortical layer) and 1.3·10 -8 g/g (medullary layer), in the bones 1·10 -9 g/g, in bone marrow 1-10 -8 g/g, in hair 1.3·10 -7 g/g. Uranium contained in bone tissue causes its constant irradiation (the half-life of Uranium from the skeleton is about 300 days). The lowest concentrations of Uranium are in the brain and heart (10 -10 g/g). The daily intake of Uranium with food and liquids is 1.9·10 -6 g, with air - 7·10 -9 g. The daily excretion of Uranium from the human body is: with urine 0.5·10 -7 - 5·10 -7 g, with feces - 1.4·10 -6 -1.8·10 -6 g, with hair - 2·10 -8 g.
According to the International Commission on Radiation Protection, the average content of Uranium in the human body is 9·10 -5 g. This value may vary for different regions. It is believed that Uranium is necessary for the normal functioning of animals and plants.
The toxic effect of uranium is determined by its chemical properties and depends on solubility: uranyl and other soluble compounds of uranium are more toxic. Poisoning by uranium and its compounds is possible at enterprises for the extraction and processing of uranium raw materials and other industrial facilities where it is used in the technological process. When it enters the body, Uranium affects all organs and tissues, being a general cellular poison. Signs of poisoning are caused by primary damage to the kidneys (the appearance of protein and sugar in the urine, subsequent oliguria); the liver and gastrointestinal tract are also affected. There are acute and chronic poisonings; the latter are characterized by gradual development and less severe symptoms. With chronic intoxication, disorders of hematopoiesis, the nervous system, etc. are possible. It is believed that the molecular mechanism of action of Uranium is associated with its ability to suppress the activity of enzymes.