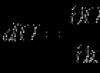Dependence of electrical conductivity of metals on temperature
In metals, the valence band is filled with electrons either partially or entirely, but overlaps with the next allowed band.
Filled states are separated from unfilled states by the Fermi level.
Thus, The Fermi level in metals is located in the allowed band.
The electron gas in a metal is practically degenerate, in this case
· electron concentration practically does not depend on temperature,
· and the temperature dependence of electrical conductivity is entirely determined by the temperature dependence of mobility.
· In the high temperature range
In metals, as well as in semiconductors, scattering of electrons by phonons dominates,
And mobility is inversely proportional to temperature.
Then the resistivity increases linearly with temperature.
· At low temperatures
The phonon concentration becomes low,
Mobility is determined by scattering by impurities and does not depend on temperature.
The resistance remains constant (Fig. 5.10).
HALL EFFECT
 The American physicist E. Hall conducted an experiment (1879) in which he passed a direct current I through a plate M made of gold and measured the potential difference between opposite points A and C on the upper and lower faces. These points lie in the same cross section of the conductor M.
The American physicist E. Hall conducted an experiment (1879) in which he passed a direct current I through a plate M made of gold and measured the potential difference between opposite points A and C on the upper and lower faces. These points lie in the same cross section of the conductor M.
Therefore, as you would expect.
When the current-carrying plate was placed in a uniform magnetic field perpendicular to its side faces, the potentials of points A and C became different. This phenomenon is called HALL EFFECT.
Fig.5.11. Consider a rectangular sample through which a current flows with density .
The sample is placed in a magnetic field with induction , perpendicular to the vector
Under the influence of an electric field, electrons in a conductor acquire a drift velocity.
The parameter that relates the drift velocity of charge carriers to the electric field strength is called carrier mobility.
Then and - mobility is numerically equal to the drift speed in an electric field of unit strength.
A particle moving at this speed in a magnetic field is acted upon by the Lorentz force, directed perpendicular to the vectors and.
Under the influence of forces, the electron moves along the sample, simultaneously rotating (under the influence of a magnetic field).
The trajectory of such movement is a cycloid.
A magnetic field in which the radius of curvature of the trajectory is much greater than the mean free path of the electron is called weak.
Under the influence of the Lorentz force, electrons are deflected to the side surface of the sample, and an excess of negative charge is created on it.
On the opposite side there is a lack of negative charge, i.e. excess of positive.
Charge separation occurs until the force acting on the electrons from the emerging electric field, directed from one side surface to the other, compensates for the Lorentz force. This field is called Hall field, and itself the phenomenon of the appearance of a transverse electric field in a sample with a current flowing through it under the influence of a magnetic field was called Hall effect .
The separation of charges will cease under the condition .
Then the potential difference between the side faces, called Hall EMF or Hall potential difference is equal to
![]() , (5.1)
, (5.1)
Where - sample width.
Current Density ,
Where n- concentration of charge carriers.
expressing the speed and substituting in (5.1), we obtain
![]() ,
,
- Hall constant.
The numerical value of the Hall constant depends on the plate material, and for some substances it is positive, and for others negative.
The sign of the Hall constant coincides with the sign of the charge of the particles that determine the conductivity of a given material.
That's why based on Hall constant measurements for a semiconductor it is possible
1. judge about the nature of its conductivity :
· If - electronic conductivity;
· If - hole conductivity;
· If both types of conductivity occur in a conductor, then by the sign of the Hall constant one can judge which of them was predominant.
2. determine the concentration of charge carriers if the nature of conductivity and their charges are known (for example, for metals. For monovalent metals, the concentration of conduction electrons coincides with the concentration of atoms).
- estimate the mean free path of electrons for an electronic conductor.
Where is the absolute value of the charge and mass of the electron;
The study of the electrical properties of materials includes the determination of electrical conductivity and its temperature dependence. For metals, the temperature coefficient of electrical conductivity is negative, that is, the electrical conductivity of metals decreases with increasing temperature.
For semiconductors and many dielectrics, the temperature coefficient of intrinsic electrical conductivity is positive. Electrical conductivity also increases with the introduction of defects and impurities into the intrinsic semiconductor.


The electrical conductivity of ionic crystals usually increases with increasing temperature and near T pl reaches the conductivity of liquid electrolytes (s NaCl at 800 °C is equal to 10 –3 Ohm –1 × cm –1), while at room temperature chemically pure NaCl is an insulator.
In crystals of alkali metal halides (for example, NaCl), cations are more mobile than anions:

Rice. 6 – Migration of cation vacancies (or Na + ions) in NaCl
Therefore, the magnitude of the ionic conductivity of NaCl depends on the number of available cation vacancies.

The number of cation vacancies, in turn, strongly depends on the chemical purity and thermal history of the crystal. An increase in the number of thermodynamically equilibrium intrinsic vacancies occurs either when the crystal is heated,
| (22) |
or when heterovalent impurities are introduced, vacancies may arise that compensate for the excess charge of impurity cations.
So, when adding small amounts of MnCl 2, NaCl + MnCl 2 ® Na 1–2 x Mn x VNa x Cl (solid solution), where for each Mn 2+ ion there is one cation vacancy associated with it, i.e. impurity vacancies (V Na) arise. Such vacancies are called impurity vacancies, since they cannot form in pure NaCl.
At low temperatures (~25 o C) the concentration of vacancies of thermal origin is very low. Therefore, despite the high purity of the crystal, the number of intrinsic vacancies remains much less than impurity ones. And with increasing temperature, a transition occurs from impurity to intrinsic conductivity.
The temperature dependence of ionic conductivity obeys the Arrhenius equation:
s = = A exp( –E a/RT), (23)
Where E a– activation energy of electrical conductivity.
The pre-exponential factor A includes several constants, including the vibration frequency of potentially mobile ions. The graphical dependence of ln s on T -1 should be expressed as a straight line with a slope angle –E/R. In some cases, when processing the temperature dependence, a factor of 1/T is introduced into the pre-exponential factor. In this case, the graphical dependence is usually presented in coordinates ln sT – T -1. The slope of the resulting straight line (E/R) may differ slightly from the slope in Arrhenius coordinates. The Arrhenius dependence for NaCl is shown schematically in Fig. 7. In the low-temperature impurity region, the number of vacancies is determined by the impurity concentration and is a constant value for each concentration level. In Fig. 7 this corresponds to a series of parallel straight lines, each of which corresponds to the conductivity of crystals with different dopant contents.

Rice. 7 – Dependence of ionic conductivity of NaCl on temperature. Parallel lines in the impurity region correspond to different concentrations of dopant impurities
In the impurity region, the dependence of s on temperature is determined only by the temperature dependence of the cation mobility m, which also obeys the Arrhenius equation:
m = m 0 exp( – E moment /RT), (23)
Where E instant is the energy of activation of carrier migration.


a NaCl = 0.564 nm; d Na - Cl = a/2 = 0.282 nm; r Na + = ~0.095 nm; r Cl - = ~ 0.185 nm.
The Na-Cl bond length, calculated as the sum of these ionic radii, turns out to be ~0.28 nm, which is close to the experimentally found value.

Rice. 8 – Migration path of Na + ion in NaCl



Rice. 9 – Triangular interstice through which the moving Na + ion in NaCl must pass. r / - radius of the inscribed circle; circles 1-3 depict Cl - ions with radius x/2.

In the impurity region (Fig. 7), the conductivity, as we see, depends on the concentration of vacancies
s = ne m 0 exp(– E moment /RT). (24)
At a higher temperature in the region of intrinsic conductivity, the concentration of vacancies of thermal origin exceeds the concentration of vacancies caused by alloying additives and the number of vacancies is already n depends on temperature according to the Arrhenius equation:
n = N×const×exp( –E arr. / 2RT). (25)
This equation is identical to equation 22, in which E arr /2R is the activation energy for the formation of one mole of cataonic vacancies, i.e., half the energy required for the formation of one mole of Schottky defects. The mobility of vacancies is still described by equation 23, and thus, in general, the electrical conductivity in the intrinsic conductivity region obeys the equation
s = N×const×m 0 exp(– E moment /RT)exp(– E arr. / 2RT)(26)
 .
(27)
.
(27)



Rice. 10 – Temperature dependence of ionic conductivity of “pure” NaCl

 Deviations from linear dependence near T pl are associated with an increase in the mobility of anion vacancies, as well as with long-range (Debye-Hückel) interactions of cation and anion vacancies, leading to a decrease in the energy of vacancy formation. Deviations from linearity in the low temperature region are determined by the formation of defect complexes that can be destroyed only at a certain activation energy.
Deviations from linear dependence near T pl are associated with an increase in the mobility of anion vacancies, as well as with long-range (Debye-Hückel) interactions of cation and anion vacancies, leading to a decrease in the energy of vacancy formation. Deviations from linearity in the low temperature region are determined by the formation of defect complexes that can be destroyed only at a certain activation energy.
In table Figure 7 shows the activation energies for conductivity of NaCl crystals.
Table 7 - Activation energy values for conductivity of NaCl crystals


The temperature dependence of electrical conductivity has been known for a long time. However, it has not been used to predict chemical processes in solids.
In 1987, a previously unknown pattern of pyrometallurgical reduction of elements from oxides was experimentally established, which consists of a simultaneous change in the type of conductivity of the oxides (from impurity to intrinsic) and their reactivity, caused by an increase in the concentration of free electrons in the crystal lattice of semiconductor oxides. In other words, the reduction of oxides begins at a temperature corresponding to the transition from impurity conductivity to intrinsic conductivity.
Dielectrics. Dielectric materials are used in electronics for the manufacture of passive elements (rigid substrates, containers, masks), as well as active elements (capacitors and electrical insulators).
Dielectrics, which include most ionic crystals, are characterized by
High electrical strength, i.e. resistance to degradation (change in structure) at high electric field strengths and transition to a conducting state;
Low dielectric losses (tgd), i.e. loss of energy from an alternating electric field, which is released in the form of heat.
The dielectric properties of materials are determined by studying flat capacitors, which are two plane-parallel conducting plates located from each other at a distance d, which is much smaller than the size of the plates (Fig. 6).

Rice. 6 – Capacitor with parallel plates and a dielectric between them
Capacity of a capacitor in a vacuum
C 0 = e 0 S/d, (28)
The dielectric constant of vacuum in the international system of physical quantities (SI) is a dimensional quantity
e 0 = 10 7 /4pс 2 = 8.854 × 10 –12 F/m. (29)
When a potential difference V is applied to the plates, the capacitor stores a charge Q o equal to
Q 0 =C 0 V. (30)
If a dielectric is placed between the plates, when the same potential difference is applied, the accumulated charge increases to Q 1, and its capacity to C 1.
For a dielectric with charge value Q 1 and capacitance C 1 dielectric constant is related to capacitance by the following relationship
e" = C 1 /C 0 . (31)
For air e" » 1;
for most ionic compounds e" ~ 5 ¸ 10;
for ferroelectrics (BTiO 3) e" = 10 3 ¸ 10 4.
e" depends on the degree of polarization or charge displacement occurring in the material.
Dielectric polarizability a is the coefficient relating the dipole moment ( R) and local electric field ( E).
p = a E, (32)
and a = a e+ a i+ a d+ a s, (33)
where a e– displacement of the electron cloud,
a i– ions,
a d– dipoles,
a s– space charge.
Electronic polarizability a e arises as a result of the displacement of the electron orbitals of atoms relative to the nuclei and is inherent in all solids. Some solids, such as diamond, have a e– the only component of polarizability;
Ionic polarizability a i– associated with the relative displacement or separation of cations and anions in a solid (determines polarization in ionic crystals);
Dipole polarizability a d– occurs in substances that have permanent electric dipoles (H 2 O, HCl), which can lengthen or change orientation under the influence of a field. At low temperatures a d frozen.
Volume charging a s occurs in “bad” dielectrics and is determined by the migration of carriers over long distances. In NaCl, migration of cations occurs along cation vacancies to the negative electrode. As a result, an electric double layer appears, which leads to an increase in e" (apparent e" appears on the order of 10 6 ... 10 7, which corresponds to the capacitance of the electric double layer (18 ... 36 μF/cm 2).
By contribution to the polarization value and dielectric constant
a s>a d>a i>a e.
These polarizability components are found from capacitive, microwave and optical measurements over a wide frequency range ( f) (Fig. 7).

|
At f < 10 3 Гц все aдают вклад в величину p.
At f> 10 6 in most ionic crystals the space charge does not have time to form.
At f> 10 9 (microwave region) there is no dipole polarization.
In area f> 10 12 , corresponding to vibrations in the optical range, the only polarization component remains a e, which is still observed in the UV region, but disappears at frequencies corresponding to the X-ray range. In good dielectrics that do not have a d and a s, the permeability at low frequency e" 0 is determined mainly by ion and electronic polarization. The value of e" 0 can be obtained from capacitance measurements using an alternating current bridge. To do this, the capacitance is measured twice - without the substance being studied between the capacitor plates and with the substance (equation 31). The quantity e" ¥, associated only with electronic polarizability, can be found from measurements of the refractive index in the visible region of the spectrum based on the simple relation e" ¥. For example, for NaCl e" 0 = 5.62; e" ¥ = 2.32.

where w = 2p f(angular frequency),
t – relaxation time (currently, the term has been introduced to describe complex polarization processes in dielectrics relaxation time distribution).
The dielectric loss tangent is determined by the relation
e // / e " = tgd(36)

Rice. 9 – Frequency dependence of e / and e //
In the area between e/0 and e / ¥ dielectric constant is represented as a complex quantity e * = e / - je // where e // is the real component, which is found from the following relationship:

where w is the angular frequency equal to 2pf, w p is the frequency of current carrier jumping, and n 1 and n 2 are constants. This equation is based on the idea that individual polarization phenomena, be it ion jumps in conductors or reorientation of dipoles in dielectrics, do not occur independently of each other, but as a result of cooperative interaction. This means that if any individual dipole in the crystal is reoriented, it thereby affects the dipoles surrounding it. At the current level of understanding, however, it is not clear how to arrive at a quantitative description of cooperative phenomena based on Jontscher's law. Diagrams in the complex plane are discussed in more detail in Chap. 13 (but at the same time, emphasis was placed on the description of conductivity, and not dielectric properties).
In table Table 8 shows the dielectric constants of some oxides at various frequencies and temperatures.
Table 8 - Dielectric constant of some oxides
| Oxide | frequency Hz | T,TO | e" | Oxide | frequency Hz | T,TO | e" |
| H 2 O (ice) | 10 8 | 3,2 | VeO | 10 5 | 6,3 | ||
| H 2 O liquid | 10 8 | 88,0 | Al2O3 | ~10 6 | 10–12 | ||
| TiO2 | 10 4 | ||||||
| H 2 O (steam) | 10 6 | 1,013 | WO 3 | ~10 8 | |||
| SiO2 | 3.10 7 | 4,3 | ZnO | 10 6 | |||
| SiO | >10 8 | 2,6...4,0 | PbO | 4,5.10 3 | |||
| Nb2O5 | ~10 12 | 35…50 | PbO2 | ~10 8 | |||
| SnO2 | ~10 12 | 9–24 | Tb4O7 | 10 6 | |||
| MnO | 4.4×10 8 | 13,8 |
The relationship between ionic and electronic polarization is a measure of the ordering of electrons relative to the ions of the crystal lattice
 . (39)
. (39)
From those given in table. 9 data it follows that even a small change in h leads to a significant change in the properties of passive elements of microelectronics ( U pr – breakdown voltage, D G 0 – free energy of formation). The higher h, the greater the electronic polarization is relative to total and the greater the possibility of controlling polarization using an electric field.
| |
| Dielectric | WITH, µF/cm | e" | tgd | U pr, V | h | –D G 0 , kJ/mol |
| at 10 3 Hz | ||||||
| Ta2O5 | 0,15 | 1,5 | 0,48 | |||
| Al2O3 | 0,085 | 1,0 | 0,49 | |||
| Al 2 (SiO 3) 3 | 0,01 | 6,5 | 0,3 | 0,50 | ||
| SiO | 0,014 | 0,1 | 0,52 | |||
| SiO2 | 0,0046 | 0,1 | 0,55 | |||
| AlN | 0,045 | 7,2 | 0,01 | – | 0,75 | |
| Si 3 N 4 | 0,04 | 6,5 | 0,001 | – | 0,94 | |
| La 2 O 3 | 0,05...1,0 | 0,02 | – | 0,60 | ||
| NaTaO 3 | 0,6 | 0,01 | – | 0,50 |
Of greatest importance for assessing the quality of dielectrics at high frequencies is the ratio between the ionic and electronic components of polarization, i.e., between and , as well as the value of the dielectric loss tangent (tgd). When alternating current passes through a capacitor at low frequencies, the current vector is 90° ahead of the voltage vector in phase. Then the product of vectors i×V = 0 and energy is transferred without loss. As the frequency increases, ionic polarization appears and the phases of current and voltage shift. In this case, a current component i×sind arises, which is in phase with the voltage.
The tgd value for high-quality dielectrics is on the order of 0.001.
For capacitors rated WITH> 50 pF tgd does not exceed 0.0015,
and with a capacitance of the order of 0.01 µF tgd ~ 0.035.
The properties of dielectrics have a significant impact on the quality of MOS structures used in microelectronics. These properties are determined by capacitance-voltage or capacitance-voltage characteristics ( C-V or VFH methods).
Ferroelectric, piezo- and pyroelectrics. The polarization of crystals belonging to centrosymmetric point groups is removed after the field is removed. However, out of 32 point groups, 21 do not contain a center of symmetry. In this regard, phenomena of residual polarization arise in electrical, mechanical and thermal fields. In accordance with these phenomena, classes of ferroelectric, piezo- and pyroelectrics are distinguished.
Ferroelectrics differ from conventional high e dielectrics " and residual polarization, that is, they have the ability to retain some residual electric polarization after removing the external electric field. Therefore, with equal volumes, capacitors made of ferroelectrics have a capacity 1000 times greater. In addition, unlike conventional dielectrics, in which there is a proportional increase in the induced polarization p or the induced charge Q (equation 30), in ferrodielectrics the relationship between the magnitude of the polarization ( R, C/cm 2) and electric field strength is characterized by hysteresis. (Fig. 11) The shape of the hysteresis determines the amount of residual polarization ( Р R) and coercive field ( N s), which removes polarization. Segentoelectrics are characterized by the presence of saturation polarization P S at high electrical voltages, for example, for BaTiO 3 P s= 0.26 C/cm 2 at 23 °C and residual polarization PR, i.e. polarization that persists after the external electric field is removed. In order to reduce the polarization to zero, it is necessary to apply an electric field E e of the opposite sign, called a coercive field.

Rice. 11 – Hysteresis loop for a typical ferrodielectric. The dashed line passing through the origin shows the behavior of a conventional dielectric.
Some of the ferroelectrics are given in Table. 10. All of them have structures in which one cation, for example, Ti 4+ in BaTiO 3, can be significantly displaced (~ 0.01 nm) relative to its anionic environment. This charge displacement leads to the appearance of dipoles and a high dielectric constant, which is characteristic of ferroelectrics.
Table 10 - Curie temperature of some ferroelectrics

In Fig. Figure 12 shows the unit cell of strontium titanate SrTiO 3, which, like BaTiO 3, has a perovskite-type structure BaTiO 3. Ti 4+ ions occupy the vertices of this cubic primitive cell, O 2– ions occupy the middle of the edges, and strontium ions occupy the center of the cube. However, you can imagine the structure of BaTiO 3 in another way: Ba 2+ ions are located at the vertices of the cube, Ti 4+ in the center, and O 2– ions in the centers of the faces. However, regardless of the choice of unit cell, the structure is constructed from TiO 6 octahedra, forming a three-dimensional framework by joining with common vertices, strontium ions in this framework structure occupy voids with CN = 12.

Rice. 12 - Structure of perovskite SrTiO 3
From a chemical point of view (the possibility of quantum chemical calculations and experimental control of dielectric properties), the perovskite structure consists of TiO 6 octahedra, and Ba 2+ ions are located in the resulting voids. In such an ideal structure, existing at temperatures above 120 °C, all charges are located symmetrically, there is no intrinsic dipole moment, and BaTiO 3 is a common dielectric with high e " . As the temperature decreases, Ti 4+ ions shift to the top of the octahedron by 0.1 Å (with an average Ti-O bond length of 1.95 Å), which is confirmed by X-ray diffraction data, i.e. distortions arise, manifested in the fact that the TiO 6 octahedra are no longer symmetrical. A dipole moment arises, and as a result of the interaction of dipoles, spontaneous polarization occurs (Fig. 13).
If such displacements occur simultaneously in all TiO 6 octahedra, then the material develops its own spontaneous polarization. In the ferroelectric BaTiO 3, each of the TiO 6 octahedra is polarized; the influence of an external electric field is reduced to the “forced” orientation of individual dipoles. After all dipoles are aligned along the field direction, a saturation polarization state is achieved. The distance by which titanium ions are displaced from the centers of the octahedra to one of the oxygens, according to estimates made on the basis of the experimentally observed value of Pa, is - 0.01 nm, which is also confirmed by X-ray diffraction analysis data. As can be seen, this distance turns out to be quite small in comparison with the average Ti-O bond length in TiO 6 octahedra, equal to 0.195 nm. The ordered orientation of the dipoles is shown schematically in Fig. 13, a, where each arrow corresponds to one distorted TiO 6 octahedron.

Rice. 13 - Scheme of orientation of the polarization vector of structural units in ferroelectrics (a), antiferroelectrics (b) ferroelectrics (c)
In ferroelectrics like BaTiO 3 , domain structures are formed due to the fact that neighboring TiO 6 dipoles spontaneously align parallel to each other (Fig. 14). The size of the resulting domains varies, but, as a rule, can reach tens to hundreds of angstroms in cross section. Within one domain, dipoles are polarized in the same crystallographic direction. The intrinsic polarization of any ferroelectric sample is equal to the vector sum of the polarizations of individual domains.

Rice. 14 – Ferroelectric domains separated by a domain wall (boundary)
The application of an external electric field leads to a change in the intrinsic polarization of the ferroelectric sample; The reason for such changes may be the following processes:
1) change in the direction of polarization of domains. This will happen if all TiO 6 dipoles within the domain under consideration change their orientation; for example, all dipoles in domain (2) (Fig. 14) change orientation to parallel to the dipoles of domain (1);
2) an increase in polarization within each domain, which is especially likely if before the application of the field there was some disorder in the orientation of the dipoles;
movement of domain walls, as a result of which the sizes of domains oriented along the field increase due to the reduction of domains with unfavorable orientation. For example, domain 1 (Fig. 14) can grow when the domain wall is shifted one step to the right. To implement such a shift, the dipoles at the boundary of domain 2 must take the orientation shown by the dashed arrows.
The ferroelectric state is usually observed at low temperatures, since thermal motion, which increases with increasing temperature, disrupts the consistent nature of the displacement in neighboring octahedra and, consequently, disrupts the domain structure. The temperature at which this destruction occurs is called the ferroelectric Curie point T a (Table 10). Above Tc, materials become paraelectrics (i.e., “non-ferroelectrics”); their dielectric constants still have high values (Fig. 15), but residual polarization is no longer observed in the absence of an external field.
Above Tc, the value of e" is usually described by the Curie-Weiss law:
e / = C/(T-q) (37)
where C is the Curie constant and q is the Curie-Weiss temperature. As a rule, Tc and q are the same or differ by only a few degrees. The transition from the ferroelectric to the paraelectric state at T c is an example of an order-disorder phase transition. However, unlike order-disorder transitions observed, say, in bronzes, there is no diffusion displacement of ions over long distances. Below T a, ordering occurs through preferential distortion or coordinated tilting of polyhedra and thus refers to phase transitions with displacement ( Ch. 12). In the high-temperature paraelectric phase, distortions and tilting of the polyhedra, if present, are in any case random.
A necessary condition for spontaneous polarization and ferroelectric properties in a crystal is that the latter must belong to a space group that does not have a center of symmetry ( Ch. 6). Paraelectric phases that are stable above Tc are often centrosymmetric, and the ordering that occurs upon cooling is reduced to a reduction in symmetry to a non-centrosymmetric space group.
Currently, several hundred ferroelectric materials are known, among which a large group of oxide compounds with a distorted (non-cubic) perovskite structure stands out. These compounds contain cations that “feel” comfortable in a distorted octahedral environment - Ti, Ni, Ta; the disparity of bonds within such distorted MO 6 octahedra is the cause of the occurrence of polarization and dipole moment. Not all perovskites are ferroelectric; for example, in contrast to BaTiO 3 and PbTiO 3, CaTiO 3 does not exhibit ferroelectric properties, which is apparently due to the difference in the sizes of doubly charged cations. The large radius of the Ba 2+ ion causes expansion of the unit cell compared to CaTiO 3 , which in turn leads to longer Ti-O bond lengths in BaTiO 3 and a greater displacement of Ti 4+ ions inside TiO 6 octahedra. Other oxides with ferroelectric properties include cations whose bonds with oxygen ions are unequal due to the presence of a free electron pair in the outer shell; these can be cations of heavy p-elemeites, corresponding to oxidation states two units less than the limit for a given group, such as Sn 2+, Pb 2+, Bi 3+, etc.
Ferroelectric oxides are used for the manufacture of capacitors due to their high dielectric constant, which is especially high near T c (Fig. 15). Therefore, pursuing the practical goal of increasing it is necessary to create materials with Curie points close to room temperature. In particular, the Curie temperature, which is 120 °C for BaTiO 3 (Fig. 15), can be significantly reduced, and the transition temperature range is expanded by partially replacing Ba 2+ or Ti 4+ with other cations: replacing Ba 2+ with Sr 2+ causes compression of the unit cell of the structure and a decrease in T c; the replacement of “active” Ti 4+ ions with other “inactive” four-charge ions, in particular Zr 4+ and Sn 4+, leads to a sharp drop in T c.

Rice. 15 – Temperature dependence of the dielectric constant of ceramic BaTiO 3
Spontaneous polarization, similar in nature to the polarization of ferroelectrics, is also observed in antiferroelectrics. Individual dipoles of antisegietoelectrics are ordered relative to each other in such a way that each dipole turns out to be parallel to neighboring dipoles (Fig. 14, b). As a result, the material’s own spontaneous polarization turns out to be zero. Above the antiferroelectric Curie point, the material becomes a normal paraelectric. Lead zirconate PbZrO 3 (233 °C), sodium niobate NaNbO 3 (638 °C) and ammonium dihydrogen phosphate NH 4 H 2 PO 4 (-125 °C) are examples of substances with antiferroelectric properties (numbers in parentheses indicate the corresponding Curie points) .
| | ¯¯¯¯¯ | |
| | ¯¯¯¯¯ | |
| | ¯¯¯¯¯ | |
| Ferroelectrics BaTiO 3 | antiferroelectrics PbZrO 3 | Ferroelectrics (Bi 4 Ti 3 O 12, tartrates) |
Rice. 16 - Scheme of orientation of the polarization vector of structural units in specific representatives of ferroelectrics (a), antiferroelectrics (b) ferroelectrics (c)
Spontaneous polarization occurs in antiferroelectrics ( P s= 0), there is no hysteresis, but near T kr also observed maximum e " .
The magnitude of the electric field strength can affect the phase
second-order transitions in ferroelectrics (Fig. 14).

Rice. 1 - Effect of temperature on orientational phase transitions
order–disorder type in PbZrO 3

Rice. 16 – Dependence of the temperature of the antiferroelectric–ferroelectric transition in PbZrO 3 on the applied voltage (a) and the behavior of polarization during this transition (b)
|  |
|
| A | b |
Rice. 17 – Structures of ferroelectric KH 2 PO 4 (a) and antiferroelectric NH 4 H 2 PO 4 (b) (projection onto a plane)
In pyroelectrics unlike ferroelectrics, the direction of the polarization vector cannot be changed by an external electric field, and polarization depends on temperature changes:
D P s = pD T, (38)
where p is the pyroelectric coefficient.
Pyroelectric properties are revealed when heated as a result of expansion of the crystal lattice and changes in the length of the dipoles. An example of a pyroelectric compound is a ZnO crystal, which contains layers of oxygen ions (hexagonal close packing) and Zn 2+ ions in tetrahedral voids. All ZnO tetrahedra are oriented in the same direction and have a dipole moment, as a result of which the crystal is in a polarized state. The pyroelectric effect is masked by water adsorption and is detected when heated.

Fig. 18 – Ordered tetrahedral structures of wurtzite. Shown is one layer of oxygen ions and the placement of Ti + cations at interstices.
Piezoelectrics also belong to non-centrosymmetric point groups of crystals. Polarization and electric charge on opposite faces of the crystal arise under the influence of mechanical fields and depend on the direction of the field. In quartz, polarization occurs upon compression along the (100) direction and is absent upon compression along the (001) axis.
Piezoelectrics There are many crystals with a tetrahedral structure, the distortion of which leads to polarization (quartz, ZnS, ZnO). A similar piezoelectric effect (PEE) is observed in La 2 S 3 . An important group of piezoelectrics consists of solid solutions of PbTiO 3 and PbZrO 3. All ferroelectrics are pyro- and piezoelectrics, but not all pyro- and piezoelectrics are ferroelectrics.

Rice. 19– Phase diagram of the PZT system
Federal Agency for Education
Volgograd State Technical University
Department of “Experimental Physics”
Study of the temperature dependence of the electrical conductivity of metals and semiconductors
Guidelines
for laboratory work No. 602
Volgograd
UDC 53 (075.5)
Study of the temperature dependence of the electrical conductivity of metals and semiconductors: method. decree. for laboratory work / comp.: V.E. Avvakumov, G.Yu. Vasilyeva; Volgograd. state tech. univ. –Volgograd, 2006. – 12 p.
Designed for students of all forms of study.
Il. 4. Table. 2. Bibliography: 3
Reviewer Assoc. A.V. Golovanov
Published by decision of the editorial and publishing council of Volgograd State Technical University
Compiled by: Vladislav Evgenievich Avakumov
Galina Yurievna Vasilyeva
Study of the temperature dependence of the electrical conductivity of metals and semiconductors
Guidelines for laboratory work No. 602
Templan 2006 pos. No.
Signed for printing. Format 60x84 1/16.
Newsprint paper. Offset printing. Conditional oven l. 1,16 .
Circulation 150 copies. Order. For free.
Volgograd State Technical University.
400131 Volgograd, prosp. them. IN AND. Lenina, 28.
RPK “Polytechnic” of Volgograd State Technical University.
400131 Volgograd, st. Sovetskaya, 35.
© Volgogradsky
state
technical
University, 2006.
602. Study of the temperature dependence of the electrical conductivity of metals and semiconductors
602.1. Goal of the work
Study of the dependence of the electrical resistance of metal conductors and semiconductors on temperature; calculation of the temperature coefficient of resistance and determination of the band gap of a semiconductor  .
.
Electrical conductivity is the ability of a body to pass electric current under the influence of an electric field. To characterize this phenomenon, use the quantity conductivity
 . Size
. Size  can be expressed in terms of the concentration of free carriers n, their charge e, mass m, time
can be expressed in terms of the concentration of free carriers n, their charge e, mass m, time  and length
and length  free run, average drift speed
free run, average drift speed  charge carriers. For a metal, free electrons act as free charge carriers. Thus, for metals
charge carriers. For a metal, free electrons act as free charge carriers. Thus, for metals
 , (602.1)
, (602.1)
Where u– mobility of carriers. Carrier mobility– a physical quantity numerically equal to the drift velocity acquired by carriers in a field of unit strength.
Depending on the  all substances are divided into conductors (σ >10 6 (Ohm m) -1), dielectrics (σ<10 -8 (Ом·м) -1)
и полупроводники (промежуточное значение
σ).
all substances are divided into conductors (σ >10 6 (Ohm m) -1), dielectrics (σ<10 -8 (Ом·м) -1)
и полупроводники (промежуточное значение
σ).
From the point of view of band theory, the division of substances into conductors, semiconductors and dielectrics is determined by how the valence band of the crystal is filled with electrons at 0 K: partially or completely.
The energy imparted to electrons even by a weak electric field is comparable in magnitude to the distances between levels in the energy band. If there are free levels in the zone, then electrons excited by an external electric field will fill them. The quantum state of the electron system will change, and a preferential (directional) movement of electrons against the field will appear in the crystal, i.e., an electric current will arise. Bodies in which similar behavior of electrons is observed are called conductors(Fig. 602.1a).
If the valence band (VB) is completely filled, then a change in the state of the electron system occurs only when they pass through the band gap (BG). Rearranging electrons inside a completely filled airspace will not cause a change in the quantum state of the system (since electrons themselves are indistinguishable). In such crystals (Fig. 602.1b), called dielectrics, the external electric field is not sufficient for the passage of electrons through the gap, i.e. appearance of electric current.

With a completely filled airspace and a small width of the airfield (  ), some electrons, under the influence of thermal excitation, can move into the conduction band (Fig. 602.1c). Such substances are called semiconductors.
), some electrons, under the influence of thermal excitation, can move into the conduction band (Fig. 602.1c). Such substances are called semiconductors.
According to expression (602.1), a change in the electrical conductivity of bodies with temperature can be caused by a change in concentration n charge carriers or a change in their mobility u.
For metals, the concentration of free charge carriers is equal to:
 , (602.2)
, (602.2)
Where  - normalized Planck constant,
- normalized Planck constant,  - Fermi energy.
- Fermi energy.
Because  is practically independent of temperature, then the concentration is also independent of temperature. Consequently, the temperature dependence of the electrical conductivity of metals is determined only by the mobility u electrons. In the high temperature range
is practically independent of temperature, then the concentration is also independent of temperature. Consequently, the temperature dependence of the electrical conductivity of metals is determined only by the mobility u electrons. In the high temperature range  , and in the low temperature region
, and in the low temperature region  .
.
The degree of mobility of charge carriers will be determined by scattering processes, i.e., the interaction of electrons with the periodic field of the lattice. Electrons can be scattered by defects in the crystal lattice (impurity atoms, structure distortions) and when interacting with phonons (thermal vibrations of the lattice).
At temperatures close to 0 K, when the intensity of thermal vibrations of the lattice and the phonon concentration are close to zero, scattering by impurities predominates ( electron-impurity scattering). In this case, the conductivity practically does not change, and the resistivity  has a constant value, which is called residual resistance
has a constant value, which is called residual resistance
 or specific impurity resistance
or specific impurity resistance
 .
.
At high temperatures, the electron-phonon scattering mechanism predominates in metals. With this scattering mechanism, electrical conductivity is inversely proportional to temperature, and resistivity is directly proportional to temperature  . Resistivity graph
. Resistivity graph  metal versus temperature is shown in Fig. 602.2a. At temperatures other than 0K and a sufficiently large amount of impurities, both electron-phonon and electron-impurity scattering can occur. Both of these scattering mechanisms are chaotic in nature. The total resistivity has the form
metal versus temperature is shown in Fig. 602.2a. At temperatures other than 0K and a sufficiently large amount of impurities, both electron-phonon and electron-impurity scattering can occur. Both of these scattering mechanisms are chaotic in nature. The total resistivity has the form  . This expression represents Matthiessen's rule about the additivity of resistance.
. This expression represents Matthiessen's rule about the additivity of resistance.

For semiconductors It was found that carrier mobility has little effect on the temperature dependence of conductivity on temperature. Then, in accordance with expression (602.1), the main contribution to the change in the electrical resistance of semiconductors should be made by a change in concentration n charge carriers.
The main feature of semiconductors is the activation nature of conductivity, i.e. a pronounced dependence of the concentration of charge carriers on external influences (temperature, irradiation, etc.). The reason for this is the small band gap (  ) in intrinsic semiconductors and the presence of additional levels in the band gap in impurity semiconductors.
) in intrinsic semiconductors and the presence of additional levels in the band gap in impurity semiconductors.
The electrical conductivity of chemically pure semiconductors is called own conductivity. The intrinsic conductivity of semiconductors arises as a result of the transition of electrons ( n) from the upper levels of the valence band to the conduction band and the formation of holes ( p) in the valence band:
Where  - concentration of electrons and holes,
- concentration of electrons and holes,  - according to their mobility, e– carrier charge.
- according to their mobility, e– carrier charge.
With increasing temperature, the concentration of electrons in the conduction band and holes in the valence band increases exponentially:
Where  - concentration of electrons and holes at
- concentration of electrons and holes at  .
.
Then, the intrinsic conductivity of semiconductors
 (602.5)
(602.5)
Where  - electrical conductivity of the semiconductor at
- electrical conductivity of the semiconductor at  ,k– Boltzmann constant. Figure 602.2b shows a graph of the dependence
,k– Boltzmann constant. Figure 602.2b shows a graph of the dependence  from return temperature
from return temperature  . The graph is a straight line, the slope of which can be used to determine the band gap
. The graph is a straight line, the slope of which can be used to determine the band gap  .
.
The electrical conductivity of doped semiconductors is due to the presence of impurity centers in it. The temperature dependence of the conductivity of such semiconductors is determined not only by the concentration of the majority carriers, but also by the concentration of carriers supplied by impurity centers. Figure 602.2c shows the dependence graphs  for semiconductors with varying degrees of doping (
for semiconductors with varying degrees of doping (  , Where n– impurity concentration). For lightly doped semiconductors, transitions involving impurity levels predominate at low temperatures. With increasing temperature, the concentration of impurity carriers and impurity conductivity increase. Upon reaching t. A (see Fig. 602.2c, curve 1) – the impurity depletion temperature
, Where n– impurity concentration). For lightly doped semiconductors, transitions involving impurity levels predominate at low temperatures. With increasing temperature, the concentration of impurity carriers and impurity conductivity increase. Upon reaching t. A (see Fig. 602.2c, curve 1) – the impurity depletion temperature  - all impurity carriers move into the conduction band. Above temperature
- all impurity carriers move into the conduction band. Above temperature  and up to the temperature of transition to intrinsic conductivity
and up to the temperature of transition to intrinsic conductivity  (t.B) electrical conductivity decreases. Above temperature
(t.B) electrical conductivity decreases. Above temperature  self-conductivity predominates, i.e. Due to thermal excitation, own charge carriers move into the conduction band. In the region of intrinsic conductivity σ
grows and ρ
- falls.
self-conductivity predominates, i.e. Due to thermal excitation, own charge carriers move into the conduction band. In the region of intrinsic conductivity σ
grows and ρ
- falls.
For heavily doped semiconductors where the impurity concentration is n ~10 26 m -3, i.e. is comparable with the concentration of charge carriers in metals (see Fig. 602.2c, curve 3), the dependence σ (T) is observed only in the region of intrinsic conductivity. With increasing concentration of impurities, the value of the interval AB (AB>A′B′>A″B″) decreases (see Fig. 602.2c). In the regions of impurity and intrinsic conductivity, the electron-phonon scattering mechanism predominates. In the region of impurity depletion (intervals AB, A′B′, A″B″) near the temperature T S is dominated by electron-impurity scattering. As the temperature increases (transition to T i) electron-phonon scattering begins to dominate. Thus, the interval AB (A′B′, A″B″), called impurity depletion region, is also the region of transition from the mechanism of impurity conductivity to the mechanism of intrinsic conductivity.
Ohm's law in differential form
contains resistivity or electrical conductivity. Specific resistance characterizes the conversion of electric current energy into heat. Current density in metal
![]() , (35.3)
, (35.3)
where is the concentration of conduction electrons, is the elementary charge, is the average speed of directional movement of electrons, is the mobility of conduction electrons, equal to the average speed of directional movement acquired by electrons under the influence of an electric field of unit strength. From (35.2) and (35.3) we obtain
![]() . (35.4)
. (35.4)
In metals, the mobility of electrons decreases with increasing temperature, since as a result of an increase in the amplitude of thermal vibrations of atoms, electrons collide with them more often, and therefore between collisions they are accelerated by an external field to lower speeds. The concentration of conduction electrons in metals does not depend on temperature. Therefore, with increasing temperature, the electrical conductivity of metals decreases, and the resistivity increases.
The specific electrical conductivity of a pure (pure) semiconductor, called own electrical conductivity,
![]() , (35.5)
, (35.5)
where , are the concentrations, and and are the mobility of conduction electrons and holes, respectively.
In pure semiconductors, the Fermi level lies approximately in the middle of the band gap. Therefore, for conduction band electrons located near the bottom of the conduction band, the exponent in (35.1)
Taking into account that , the probability of electrons filling the states of the conduction band
The number of electrons transferred to the conduction band, and therefore the number of holes formed in the valence band, will be proportional to probability (35.7).
In semiconductors, as well as in metals, the mobility of electrons and holes increases with increasing temperature, but the concentration of carriers due to the transition of more and more electrons from the valence band to the conduction band grows much faster. As a result, the electrical conductivity of the semiconductor increases:
 where is the base of natural logarithms, is the band gap, is Boltzmann’s constant, is the absolute temperature, is the limiting value of the specific electrical conductivity of a semiconductor when the temperature goes to infinity, when the populations of the valence band and conduction band by electrons are practically equalized. Thus, the electrical conductivity of a semiconductor increases exponentially with increasing temperature (see Fig. 35.10).
where is the base of natural logarithms, is the band gap, is Boltzmann’s constant, is the absolute temperature, is the limiting value of the specific electrical conductivity of a semiconductor when the temperature goes to infinity, when the populations of the valence band and conduction band by electrons are practically equalized. Thus, the electrical conductivity of a semiconductor increases exponentially with increasing temperature (see Fig. 35.10).
The temperature dependence of the semiconductor resistance has the form:
where is the limiting value of the semiconductor resistance when the temperature goes to infinity. At low temperatures, the resistivity of a semiconductor is very high and it is practically an insulator, and at very high temperatures the resistivity becomes almost the same as that of metals.
Semiconductors include crystals of many elements of the periodic table (silicon Si, germanium Ge, selenium Se, etc.), cuprous oxide, lead sulfide and many other chemical elements. Modern microelectronics is almost entirely based on silicon. The silicon atom has an atomic number in the periodic table of Mendeleev. Therefore, the charge of the nucleus of a silicon atom is equal and the atom contains 14 electrons. Four of them form the electron shell farthest from the nucleus. These four electrons are relatively weakly bound to the nucleus. They provide four covalent silicon bonds in chemical compounds and are therefore called valence electrons. The remaining ten electrons, together with the nucleus, form the core of the atom, which has a charge . Four valence electrons move around the core and form a cloud of negative charge. In Fig. Figure 35.11 shows a schematic representation of a silicon atom with its four covalent bonds.
 In the silicon crystal lattice, each atom is surrounded by four nearest neighbors. A simplified flat diagram of the arrangement of atoms is shown in Fig. 35.12. The connection between two neighboring atoms is carried out by a pair of electrons, providing the so-called pair-electron, or covalent bond. The picture shown is of pure silicon at very low temperatures. In this case, all valence electrons are involved in the formation of bonds between atoms and cannot take part in electrical conductivity.
In the silicon crystal lattice, each atom is surrounded by four nearest neighbors. A simplified flat diagram of the arrangement of atoms is shown in Fig. 35.12. The connection between two neighboring atoms is carried out by a pair of electrons, providing the so-called pair-electron, or covalent bond. The picture shown is of pure silicon at very low temperatures. In this case, all valence electrons are involved in the formation of bonds between atoms and cannot take part in electrical conductivity.
 As the temperature of the crystal increases, thermal vibrations of the lattice lead to the breaking of some covalent bonds. As a result, some of the electrons previously involved in the formation of covalent bonds are split off and become conduction electrons. In the presence of an external electric field, they move against the field and create an electric current.
As the temperature of the crystal increases, thermal vibrations of the lattice lead to the breaking of some covalent bonds. As a result, some of the electrons previously involved in the formation of covalent bonds are split off and become conduction electrons. In the presence of an external electric field, they move against the field and create an electric current.
The departure of an electron that previously took part in the formation of a covalent bond leads to the appearance of a vacancy - “ holes” (see Fig. 35.13). The appearance of holes creates an additional opportunity for charge transfer. Indeed, in the presence of a hole, the valence electron of a neighboring atom under the influence of an external electric field can move to the place of the hole. Then the covalent bond will be restored in this place, but a hole will appear in the position from which the valence electron moved, filling the vacancy. A valence electron from another neighboring atom will be able to move into this new hole, etc. As a result, the current will be supported not only by conduction electrons, but also by valence electrons, which move in the same way as conduction electrons, against the electric field. The holes will move in the direction of the electric field, that is, in the same way as positively charged particles would move. Thus, two types of electrical conductivity are possible in semiconductors: electronic, carried out by the movement of conduction electrons, and holey, caused by the movement of holes.
Along with electron transitions from a bound state to a free state (from the valence band to the conduction band), reverse transitions also occur when a conduction electron fills one of the vacancies and turns into a valence electron (returns from the conduction band to the valence band). This process is called recombination electron and hole. In an equilibrium state, such a concentration of electrons (and exactly the same concentration of holes) is established at which the same number of forward and reverse transitions occur per unit time.
The electrical conductivity of any material is determined by the concentration and mobility of free charge carriers, the values of which depend on temperature.
Mobility m of free charge carriers characterizes their scattering and is defined as the coefficient of proportionality between the drift velocity v dr and electric field strength e: v dr = m e.
Scattering free charge carriers, i.e. a change in their speed or direction of movement may occur due to the presence of structural defects in real semiconductor crystals (this includes, for example, impurity atoms and ions), and thermal vibrations of the crystal lattice.
It has been established that when charge carriers are scattered only by impurity ions, the mobility
The increase in the mobility of free charge carriers with increasing temperature is explained by the fact that the higher the temperature, the greater the thermal speed of movement of the free carrier and the less time it will be in the Coulomb field of the ion, which changes the trajectory of its movement, which means it will have less scattering and more high mobility. As the temperature rises, scattering by thermal vibrations of the crystal lattice becomes increasingly important, and at a certain temperature it becomes predominant.
Thermal vibrations of the crystal lattice increase with increasing temperature, carrier scattering also increases, and their mobility decreases. It has been established that in atomic semiconductors, when free charge carriers are scattered predominantly by thermal vibrations of the lattice
In Fig. Figure 4.10 shows the dependences of the mobility of free charge carriers in an n-type semiconductor with different donor impurity concentrations. With increasing temperature, when scattering on impurity ions, the mobility increases, and then, due to ever-increasing vibrations of the crystal lattice and the scattering caused by them, it decreases. The magnitude and position of the maximum of the m(T -1) curve depend on the impurity concentration. As it increases, the maximum shifts to the region of higher temperatures, and the entire curve moves down along the ordinate axis. At an impurity concentration equal to N D3, corresponding to a degenerate semiconductor, the mobility decreases with increasing temperature, similar to what happens in conductor materials (Section 3.8).
| |

Rice. 4.10. Dependence of the mobility of free electrons on temperature in an n-type semiconductor: N D1 At very low temperatures, when thermal vibrations of the crystal lattice are small and impurity atoms are weakly ionized, scattering of free carriers mainly occurs on neutral impurity atoms. With this scattering mechanism, mobility does not depend on temperature, but is determined by the impurity concentration. So, the concentration of free charge carriers in semiconductors increases with increasing temperature according to an exponential law, and the temperature dependence of mobility has, in general, the character of a curve with a maximum and a power law of change. In the general case, the specific electrical conductivity s of a semiconductor, in which the charge carriers are free electrons with mobility m n and free holes with mobility m p, is equal to: where e is the elementary charge. For native semiconductor Considering that the power-law dependence is weaker than the exponential one, we can write: Similarly for an n-type impurity semiconductor in the region of impurity conductivity: Relations (4.14) and (4.15) are valid only until complete ionization of the impurity occurs. Having obtained the experimental dependence of specific conductivity on temperature in the form lns(T -1), it is possible to determine the band gap of the semiconductor and the ionization energy of the impurity using relations (4.13) – (4.15). Let us consider the experimental curves of the temperature dependence of the electrical conductivity of silicon containing different amounts of donor impurity (Fig. 4.11). The increase in the specific conductivity of silicon with increasing temperature in the low temperature region is due to an increase in the concentration of free charge carriers - electrons due to the ionization of the donor impurity. With a further increase in temperature, a region of impurity depletion occurs—its complete ionization. The intrinsic electrical conductivity of silicon has not yet manifested itself noticeably. Under conditions of impurity depletion, the concentration of free charge carriers practically does not depend on temperature, and the temperature dependence of the specific conductivity of the semiconductor is determined by the dependence of carrier mobility on temperature. The decrease in silicon conductivity observed in this region with increasing temperature occurs due to a decrease in mobility when free charge carriers are scattered by thermal vibrations of the crystal lattice. Rice. 4.11. Temperature dependence of the electrical conductivity of silicon containing different amounts of donor impurity N D: 1 – 4.8×10 23 ; 2 – 2.7×10 24 ; 3 – 4.7×10 25 m -3 However, a case is also possible when the impurity depletion region is in the temperature range where the main scattering mechanism is scattering on impurity ions. Then the specific conductivity of the semiconductor will increase with increasing temperature: s~T 3/2. A sharp increase in specific conductivity with a further increase in temperature (Fig. 4.11) corresponds to the region of intrinsic electrical conductivity, in which the concentration increases exponentially [relation (4.4)], and mobility decreases according to the power law (4.10). In a degenerate semiconductor (curve 3 in Fig. 4.11), the concentration of free charge carriers does not depend on temperature and the temperature dependence of conductivity is determined by the dependence of their mobility on temperature (Fig. 4.10). 4.6. Optical and photoelectric phenomena Light absorption. Due to the reflection and absorption of light by a semiconductor, the intensity of monochromatic radiation incident on it with intensity I 0 decreases to a certain value I. In accordance with the Lambert–Bouguer law: where R is the reflection coefficient, x is the distance from the surface of the semiconductor along the direction of the beam (in the volume) to a given point; a is the absorption coefficient. The value of a -1 is equal to the thickness of the layer of substance, when passing through which the intensity of light decreases by e times (e is the base of the natural logarithm). The absorption of electromagnetic radiation energy by a semiconductor can be associated with various physical processes: disruption of covalent bonds between atoms of the material with the transition of electrons from the valence band to the conduction band; ionization of impurity atoms and the appearance of additional free electrons or holes; a change in the vibrational energy of lattice atoms; formation of excitons, etc. If the absorption of light by a semiconductor is due to the transitions of electrons from the valence band to the conduction band due to the energy of radiation quanta, then absorption is called own; if the emergence of free carriers due to the ionization of impurity atoms (donors or acceptors) – impurity. In a number of semiconductors, due to the absorption of a light quantum, it is possible to excite an electron in the valence band in such a way that it is not accompanied by its transition to the conduction band, but a coupled electron-hole system is formed, moving within the crystal as a single whole. This system is called exciton. The optical absorption of a semiconductor, caused by the interaction of radiation with the vibrational motion of the crystal lattice, is called lattice. Regardless of the mechanism of absorption of radiation quanta, the process obeys the law of conservation of energy. Photoconductivity semiconductors is a phenomenon that always accompanies the process of absorption of electromagnetic radiation energy. When a semiconductor is illuminated, the concentration of free charge carriers in it can increase due to carriers excited by absorbed light quanta. Such carriers can be either their own electrons and holes, or carriers that have passed into a free state due to the ionization of impurity atoms. Illumination of a semiconductor with light for a sufficiently long time does not lead to an infinite increase in the concentration of excess (compared to equilibrium) charge carriers, since as the concentration of free carriers increases, the probability of their recombination increases. There comes a moment when recombination balances the process of generation of free carriers and an equilibrium state of the semiconductor is established with a higher conductivity s equal to that without illumination (s 0). Rice. 4.13. Absorption spectrum of a semiconductor and spectral distribution of photosensitivity: 1 – intrinsic absorption; 2 – impurity absorption; 3.4 – photocurrent With longer wavelength radiation, when the energy of light quanta E Ф is low (E f =hn, where h is Planck’s constant, n is the frequency), at l pr impurity absorption occurs and photoconductivity (photocurrent) occurs due to the ionization of impurities (curves 2, 4 , Fig. 4.13). At a shorter wavelength l i , i.e. With a higher energy of light quanta, commensurate with the band gap of the semiconductor DE 0, intrinsic (fundamental) absorption and photoconductivity (photocurrent) arise (curves 1.3, Fig. 4.13). This wavelength l i is called the intrinsic (fundamental) absorption edge of the semiconductor. The short-wavelength decline in photoconductivity (curve 3, Fig. 4.13) is explained by the high absorption coefficient (curve 1, Fig. 4.13), i.e. Almost all light is absorbed in a very thin surface layer of the material. As stated above, photoconductivity caused by the generation of free carriers is always accompanied by the absorption of electromagnetic radiation energy. In the process of recombination, on the contrary, energy is released. The released energy can be absorbed by the crystal lattice ( nonradiative recombination) or be emitted in the form of a light quantum ( radiative recombination). The latter phenomenon has found application in LEDs used in instrument making as light indicators.![]() , (4.11)
, (4.11) . (4.13)
. (4.13) . (4.15)
. (4.15)
in semiconductors