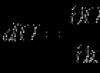When studying the coagulation of sols, many theories arose with the help of which they tried to explain all the observed patterns at the qualitative and quantitative levels.
Thus, in 1908, G. Freundlich formulated the main provisions adsorption theory of coagulation observed when adding electrolytes to the sol. According to this theory, aggregation of colloidal particles occurs due to the adsorption of counterions by the surface of the granule and a decrease in the value of its zeta potential. However, this theory had limited application, because took into account only the influence of electrolytes and could not explain those facts in which the adhesion of particles was associated only with changes in the diffuse layer of the micelle, and the value of the ζ-potential of the granule remained unchanged.
Later it was developed by G. Muller electrostatic theory, which already proceeded from the fact that the introduction of an electrolyte into a sol does not change the total charge in the electrical double layer of the particle, but causes compression (reduction in size) of the diffuse layer. This leads to a decrease in the stability of the system.
Adsorption, electrostatic and a number of other theories of coagulation could not explain all the observed experimental facts, but they played a positive role in the development of ideas about the stability of colloidal systems. Their most important provisions have become part of the modern theory of stability, which is in good agreement with the behavior of typical lyophobic disperse systems.
This theory was developed in 1937-1943. independently of each other B.V. Deryagin and L.D. Landau in the USSR and E. Verwey and J. T. Overbeck in Holland. In accordance with the first letters of the authors' surnames, the theory is called DLFO.
According to this theory, colloidal particles in a solution, due to Brownian motion, can easily approach each other until they come into contact with their liquid diffuse shells or layers. In this case, no interaction forces arise between them. For further approach, the particles must deform their diffuse shells so that they overlap (or penetrate each other). But liquids do not compress well, and in response to deformation, so-called disjoining pressure forces, preventing the implementation of this process. Moreover, the larger the dimensions of the diffuse layer, the greater the disjoining pressure forces.
Boris Vladimirovich Deryagin (1902 – 1994)– Russian physical chemist, professor (1935), corresponding member of the USSR Academy of Sciences (1946), academician of the Russian Academy of Sciences (1992). He created the doctrine of surface forces and their influence on disjoining pressure and the properties of thin liquid films. Prize named after M. V. Lomonosov Academy of Sciences of the USSR (1958), State Prize of the USSR (1991). From 1936 to 1994, he headed the laboratory and the Department of Sorption Processes at the Institute of Physical Chemistry of the USSR Academy of Sciences, which he created. For many years he was the editor-in-chief of the journal Colloidal Chemistry. In 1962 – 1973 assumed the existence of a special type of water - polywater. Then he refuted himself by discovering the critical influence of impurities - silicates.
If the colliding particles have a sufficient supply of kinetic energy to overcome the action of these forces, then their diffuse layers will overlap, but at this moment electrostatic repulsion forces will arise between them and the granules (since they have charges of the same sign) (Fig. 68).

Rice. 68. Scheme of interaction of colloidal particles: A– aggregatively stable system; b– overlapping of diffuse layers; V– coagulation
L  Ev Davidovich Landau (1908 – 1968), often referred to as Dau - Soviet physicist, academician of the USSR Academy of Sciences (elected in 1946). Laureate of the Nobel, Lenin and three Stalin Prizes, Hero of Socialist Labor. Member of the Academies of Sciences of Denmark, the Netherlands, the USA, France, the London Physical Society and the Royal Society of London. Initiator of the creation and co-author of the Course of Theoretical Physics, which has gone through multiple editions and translated into many languages. A gold medal, awarded since 1998 by the Department of Nuclear Physics of the Russian Academy of Sciences, is named after Landau.
Ev Davidovich Landau (1908 – 1968), often referred to as Dau - Soviet physicist, academician of the USSR Academy of Sciences (elected in 1946). Laureate of the Nobel, Lenin and three Stalin Prizes, Hero of Socialist Labor. Member of the Academies of Sciences of Denmark, the Netherlands, the USA, France, the London Physical Society and the Royal Society of London. Initiator of the creation and co-author of the Course of Theoretical Physics, which has gone through multiple editions and translated into many languages. A gold medal, awarded since 1998 by the Department of Nuclear Physics of the Russian Academy of Sciences, is named after Landau.
The higher the ζ-potential of the granules, the stronger the mutual repulsion of particles.
In the case of overcoming these forces and bringing the granules closer to a distance of ≈ 10–7 cm or less (i.e., a distance equal to or less than the size of one molecule of the dispersion medium), the so-called van der Waals forces of attraction, which have a physical nature, arise between them . They cause adhesion (connection) of colloidal particles to each other.
Typically, in a stabilized hydrophobic ash, only a small fraction of the so-called active particles have a sufficient supply of kinetic energy to overcome all the above forces upon impact. Therefore, such colloidal systems retain their stability for a more or less long time (depending on the degree of their stabilization). As the temperature rises, the speed and intensity of Brownian motion increase. This leads to an increase in the reserve of kinetic energy of colloidal particles. An increasing number of them are becoming active. As a result, upon collision, they will more often begin to stick to each other, and the aggregative stability of the sol will decrease.
Any other external influences exerted on the sol and leading to a decrease in the size of the diffuse layers and the value of the ζ-potential will also contribute to the occurrence of coagulation processes.
The least stable are colloidal systems, the particles of the dispersed phase in which do not have a double electrical layer and a protective shell of solvent molecules.
In this case, electrostatic repulsion forces and disjoining pressure forces do not arise between the particles and therefore almost any collision of them with each other will lead to mutual adhesion.
The physical theory of DLFO coagulation has a large mathematical apparatus and allows for various quantitative calculations that are in good agreement with the observed experimental facts.
The elementary act of coagulation occurs as a result of “short-range interaction” of particles. The precipitation is dense and irreversible, since the energy of attraction is much greater than the energy of repulsion. Here there is direct contact between particles; at distances corresponding to the first minimum, condensation-crystallization structures or coarse dispersions are formed. 2. If the height of the barrier is large and the depth of the second minimum is small, the particles cannot overcome the barrier and disperse without interaction. This is the case of an “aggregately stable system”. This stability can be broken in two ways. a) An increase in the kinetic energy of particles leads to an increase in the number of collisions. If the energy of fast particles exceeds the potential barrier, then the particles can stick together. Therefore, an increase in temperature can lead to coagulation of the system. b) The potential barrier can be reduced by adding electrolytes to the system. In this case, the EDL is compressed due to the compression of the diffuse part, as a result of which the particles approach each other at shorter distances, where the attractive forces increase. Fig. 4.3 Diagram of the influence of electrolyte on coagulation: h2< h1 3. Если глубина второго минимума достаточно велика то, незави- симо от высоты барьера, происходит так называемое «дальнее взаимо- действие» двух частиц, отвечающее второму минимуму. Вторичный минимум на участке ВС отвечает притяжению частиц через прослойку среды. Возникает взаимодействие на дальних расстоя- ниях, осадки получаются рыхлыми и обратимыми, так как минимум не глубокий. Второму минимуму соответствует явление флокуляции или образо- вание коагуляционных структур. Интерес к этим системам в последнее время велик: фиксация час- тиц во втором минимуме при достаточной концентрации дисперсной фазы может привести к превращении. Золя в полностью структуриро- ванную систему. Реальные твердые тела, составляющие основу материальной куль- туры человечества (строительные материалы, деревянные изделия, оде- жда, бумага, полимеры) – в подавляющем большинстве являются струк- турированными дисперсными системами. Вывод: Рассмотренный классический вариант теории Дерягина-Ландау да- ет хорошее согласие с экспериментальными данными. Но может быть самым главным ее достижением является обоснование правила Шульце- Гарди, которое справедливо считается краеугольным камнем для про- верки теорий устойчивости. const g = 6 – «закон шестой степени» Дерягина, устанавливающий Z зависимость порога коагуляции от заряда иона-коагулятора. 4.7 Зависимость скорости коагуляции от концентрации электролита. Медленная и быстрая коагуляция Медленная коагуляция – это когда электролита введено в таком количестве, что небольшой барьер отталкивания сохраняется (DU), здесь не все сталкивающие частицы коагулируют. Скорость ее зависит от концентрации электролита. Быстрая коагуляция – имеет место при полном исчезновении энергетического барьера, здесь каждое столкновение частиц приводит к коагуляции. Скорость быстрой коагуляции u – не зависит от концен- трации электролита. Рис.4.4 Зависимость скорости коагуляции от концентрации электролита При небольших количествах электролита скорость коагуляции близка к нулю (участок I). Затем скорость растет при увеличении количества электролита (участок II). Коагуляция на участке II является медленной и зависит от концентрации электролита. На участке III скорость достигает максимальное значение и уже не зависит от количества прибавляемого электролита. Такая коагуляция называется быстрой и соответствует полному исчезновению потенци- ального барьера коагуляции DU . Начало участка III отвечает порогу быстрой коагуляции g б, здесь величина x -потенциала падает до нуля. Порогу быстрой коагуляции на основании теории ДЛФО можно дать строгое определение: Порог быстрой коагуляции – это количество электролита, необхо- димое для снижения энергетического барьера до нуля. 4.8 Изменение агрегативной устойчивости при помощи электролитов. Концентрационная и нейтрализационная коагуляция Одним из способов изменения агрегативной устойчивости золей является введение электролитов. Электролиты в состоянии изменить структуру ДЭС и его диффуз- ный слой, снизить или увеличить x -потенциал и электростатическое от- талкивание, т.е. способны вызвать или предотвратить коагуляцию. Воз- можны концентрационная и нейтрализационная коагуляция электроли- тами. Причина их одна и та же – снижение x -потенциала, ослабление электростатического отталкивания. Однако механизм снижения x - потенциала различный. Рис.4.5 Падение потенциала в ДЭС до (кривая 1) и после (кривая 2) введения электролита в процессе концентрационной (а) и нейтрализационной (б) коагуляции j1 и j 2 , x1 и x 2 – значения полного и электрокинетического по- тенциалов, соответственно, до и после введения электролитов; 3 и 4 – направления адсорбции ионов электролита; х – расстояние от твердой поверхности в глубь жидкости. 1. Концентрационная коагуляция наблюдается при больших заря- дах поверхности, когда j0 ³ 100 мВ, и проводится она в основном ин- дифферентными электролитами. Эти электролиты способствуют сжа- тию диффузной части ДЭС, снижению x -потенциала (x 2 < x1), но не изменяют полный потенциал j0 . Благодаря этому (сжатию ДЭС) частицы сближаются и межмоле- кулярные силы притяжения начинают превалировать, что и вызывает слияние частиц. Правило Шульце-Гарди подтвердили теоретически Б.В. Дерягин и Л.Д. Ландау, представив расклинивающее давление как суммарный эф- фект сил отталкивания и притяжения, что позволило им вывести урав- нение, связывающее порог коагуляции с зарядом иона-коагулятора. B * e (kб T) 5 Cкр = g = , (1) A2 e 6 Z 6 где B * – константа; e – диэлектрическая постоянная; kб – константа Больцмана; T – абсолютная температура; A – постоянная Ван-дер- Ваальса; e – заряд электрона; Z – заряд иона-коагулятора. Это уравнение (4) хорошо описывает зависимость порога коагуля- ции от заряда иона-коагулятора для сильно заряженных поверхностей и соответствует эмпирическому правилу Шульце-Гарди. В уравнение (1) не входит потенциал поверхности. Таким образом, правило Шульце-Гарди справедливо в случае концентрационной коагу- ляции. 2. Нейтрализационная коагуляция происходит при малых потен- циалах поверхности (j0 £ 100 м В) под действием неиндифферентных, т.е. родственных электролитов. Особенно эффективны электролиты, со- держащие ионы большого заряда и большого радиуса, то есть хорошо адсорбирующиеся. При введении таких электролитов идет частичная нейтрализация полного потенциала поверхности при адсорбции противоионов, что приводит к снижению не только полного потенциала j0 , но и j " и x - потенциала, а также к сжатию диффузной части ДЭС. Для случая нейтрализационной коагуляции при j0 £ 100 м В авторы теории ДЛФО нашли выражение для порога коагуляции: " x 4 Cкр = g = k 2 . (2) Z Из уравнения (2) следует, что для нейтрализационной коагуляции критическая концентрация зависит от x -потенциала и, следовательно, от полного потенциала поверхности j0 . Из уравнения (2) также следует: при малых j0 порог коагуляции обратно пропорционален Z 2 коагулирующего иона. Этот случай соответствует эмпирическому правилу Эйлерса- Корфа, которое оказывается справедливым для слабо заряженных по- верхностей. В реальных системах одновременно могут действовать оба меха- низма коагуляции, поэтому зависимость порога коагуляции от заряда иона-коагулятора оказывается промежуточной. 4.9 Особые явления при коагуляции. Явление неправильных рядов Коагулирующая сила ионов зависит не только от заряда и радиуса коагулирующих ионов, но и от их специфической адсорбции. Кроме того, многовалентные ионы могут вызвать перезарядку по- верхности и привести к чередованию зон устойчивого и неустойчивого состояния системы. Это явление получило название явления неправиль- ных рядов. Суть: при добавлении электролитов вначале наблюдается ус- тойчивость золя, затем – коагуляция. Далее – вновь устойчивость, и, на- конец, при избытке электролита – опять коагуляция. Это объясняется тем, что многовалентные ионы (Fe3+, Al3+, Th4+) перезаряжают частицы и переводят систему из неустойчивого в устой- чивое состояние. Введение электролита AlCl3 в золь сернистого мышь- яка, имеющего первоначально отрицательный заряд. Рис.4.6 Схема неправильных рядов На рис. 4.6 можно выделить две зоны устойчивого состояния (0-1, 2-3) и две зоны коагуляции (1-2, 3-4). Зона 0-1 – электролита добавлено недостаточно, устойчивое со- стояние. Зона 1-2 – электролита добавлено достаточно, x = xкр. Идет коагу- ляция. Далее начинается перезарядка поверхности, x -потенциал приоб- ретает противоположное значение. При достижении x >+ xcr a stable state occurs again (section 2-3). At section 3-4, the system is coagulated again according to the concentrated coagulation scheme. Unlike section 1-2, where coagulation occurs with Al3+ ions, in zone 3-4 coagulation is carried out with Cl– ions, since the charge of the particles has become positive. 4.10 Coagulation with a mixture of electrolytes In industrial conditions, not one electrolyte is used for coagulation, but a mixture of several electrolytes. The coagulating effect of a mixture of two electrolytes is often non-additive. Sometimes more than one of them is required in the mixture of electrolytes - this is the phenomenon of antagonism. If a mixture of electrolytes is more effective than one electrolyte, then the phenomenon of synergism appears; less of them are needed in the mixture than of each individually. With additive action, electrolytes coagulate independently of each other. To characterize a mixture of two electrolytes, it is convenient to use a graph of the dependence of the coagulation threshold g 1 on the coagulation threshold g 2 . With additive action, the dependence g 1 – g 2 is linear. Synergism is characterized by curve 2, if the first electrolyte is taken in the amount of g 1 / 2, then the second - in the amount of g 2< g 2 / 2 . Рис.4.7 График зависимости порога коагуляции: 1 – аддитивное действие; 2 – синергетическое действие; 3 – антагонистическое действие Синергизм электролитов широко используют на практике для коа- гуляции больших количеств дисперсных систем. 4.11 Применение коагулянтов и флокулянтов в процессах очистки воды Явление коагуляции тесно связано с проблемой удаления загрязне- ний из водных сред. В основе многих методов очистки от в.д.с – загрязнений лежит яв- ление потери системой агрегативной устойчивости путем объединения частиц под внесением специально вводимых реагентов: коагулянтов и флокулянтов. Это укрупнение частиц приводит к потере седиментационной ус- тойчивости системы и образованию осадков. В настоящее время подбор реагентов для коагуляции основывается преимущественно на эмпирических исследованиях. Чаще всего коагулирование загрязнений воды производится элек- тролитами, которые содержат многозарядные ионы (Al3+, Fe3+). Ранее процесс осветления воды объясняли нейтрализацией много- валентными катионами, заряженных, как правило, отрицательно, частиц природных вод. Однако коагуляция эти ионами связана с процессами их гидролиза, в результате которого возникают полиядерные аквагидро- комплексы, обладающие более сильной коагулирующей способностью, чем ионы. Сам процесс коагуляции подобен процессу флокуляции ВМС. В процессах водоочистки постепенно расширяется применение по- лимерных флокулянтов (ВМС): длинная молекула полимера адсорбиру- ется двумя концами на двух разных частицах дисперсной фазы и соеди- няет их «мостиком». Получается рыхлый агрегат – флоккула. Здесь час- тицы не имеют непосредственного контакта между собой. Флокулянты бывают природными и синтетическими, неионоген- ными и ионогенными. В последнем случае флокуляция возможна не только по механизму мостикообразования, но и путем нейтрализации заряда частиц противоположно заряженными ионами полиэлектролита. На празднике часто эффективным оказывается совместное приме- нение коагулянтов и флокулянтов. 4.12 Кинетика коагуляции Процесс коагуляции протекает во времени. Отсюда вытекает пред- ставление о скорости коагуляции. Скорость коагуляции – это измене- ние частичной концентрации в единице объема в единицу времени. Раз- личают быструю коагуляцию, когда каждое столкновение частиц при- водит к их слипанию и медленную коагуляцию, если не все столкновения частиц являются эффективными. Термины «быстрая» и «медленная» коагуляции условны и не связаны со скоростью процесса. При опреде- ленных условиях быстрая коагуляция может протекать очень медленно и, наоборот, медленная коагуляция может идти весьма быстро. Теория кинетики быстрой коагуляции предложена С. Смолуховским. Скорость процесса уменьшения общего числа частиц (n) во времени он рассматривает как скорость реакции второго порядка, поскольку слипание частиц происходит при столкновении двух частиц, dn = k × n2 . (3) dt После интегрирования этого уравнения получим 1æ1 1 ö k= ç - ÷ (4) t è n n0 ø или n0 n= , (5) 1+ kn0t где n0 – общее число частиц в единице объема золя до коагуляции, n – число частиц к моменту времени t, k – константа скорости процесса коагуляции, которая вычисляется по уравнению (5.5). Константа k свя- зана с коэффициентом диффузии частиц D и с расстоянием d, на кото- ром действуют силы притяжения между частицами, уравнением k = 4pDd . (6) Подставив в это уравнение вместо D его значение из уравнения Эйнштейна и учитывая, что d = 2r, получим 4 RT 3 –1 k= ,м с. (7) 3h Из формулы (7) видно, что величина k не зависит от начальной концентрации золя и от размера частиц и поэтому не меняется при их слипании. Константа скорости процесса коагуляции – постоянная толь- ко для данной коллоидной системы. Если величина константы k, вычис- ленная из экспериментальных данных, не совпадает с величиной, полу- ченной из теоретической формулы (7), то это значит, что в системе про- исходит не быстрая, а медленная коагуляция. С. Смолуховский предложил формулы, позволяющие определить с к о л ь к о ч а с т и ц того или иного порядка (первичных, вторичных и т.д.) имеется в золе ко времени t. Причем для того, чтобы исключить входящие в эти формулы трудно определяемые величины D и d, он ввел в них так называемое время половинной коагуляции q (период коагуля- ции), за которое начальная концентрация первичных частиц уменьшает- ся вдвое. Тогда для первичных частиц n0 n1 = , (8) (1 + t q) 2 для вторичных частиц n0 t q n2 = (9) (1 + t q) 3 и для частиц m-го порядка n0 (t q) m-1 nm = . (10) (1 + t q) m+1 На рис. 4.8 уравнения (8-10) изображены графически. Получен- ные кривые наглядно показывают распределение числа частиц в бы- стро коагулирующем золе. В на- чальный момент, т. е. когда t = 0, все частицы – первичные: n = n1 = n0, а n2 = n3 = n4 = 0. Через некоторое время количество всех частиц равно n, число первичных n1 уменьшается, но начинают появ- ляться двойные, тройные и др. час- тицы. По мере коагуляции эти час- тицы также постепенно исчезают, уступая место частицам высших порядков – более крупным агрега- там. Поэтому кривые, выражающие Рис.4.8 Распределение числа частиц при изменение числа частиц различных быстрой коагуляции золя порядков, со временем приобрета- ют ясно выраженные максимумы. Кривые, выражающие распределение числа частиц во времени, строят также в координатах n = f (t / q) , n = f (t) или в линейной форме – в координатах 1 / n = f (t) . Согласно теории С. Смолуховского, время половинной коагуляции не зависит от времени коагуляции. Чтобы проверить применимость тео- рии, по экспериментальным данным вычисляют q для нескольких зна- чений t по формуле, полученной из (4), . (11) Если величина q не остается постоянной при различных t, то это означает, что в системе происходит не быстрая, а медленная коагуля- ция. 4.13 Примеры коагуляции. Образование почв Мы рассмотрели развитие основных идей, определяющих содержа- ние проблемы устойчивости. Так, одна из важнейших задач заключается в сохранении устойчивого состояния суспензий, эмульсий и других объектов, проходящих в процессе переработки через сложные системы производственных агрегатов. Не менее важной для народного хозяйства является и обратная задача – скорейшего разрушения дисперсных сис- тем: дымов, туманов, эмульсий, промышленных и сточных вод. Огра- ничимся здесь иллюстрацией многообразия и сложности коагуляцион- ных явлений на примерах, связанных с процессами почвообразования. Почвы образуются при разрушении горных пород в результате вы- ветривания, выщелачивания, гидролиза и т. д. Эти процессы приводят к образованию окислов: как нерастворимых, типа SiO2, Al2O3, Fe2O3 (точ- нее – их гидроокисей), так и растворимых, типа RO и R2O (где R – ме- талл). Из-за значительной гидратации нерастворимых элементов почвы и дальнему взаимодействию в процессе взаимной коагуляции образуют- ся структурированные коагуляты, близкие по свойствам к гелям, назы- ваемые коагелями. Эти коллоидно-химические процессы определяют все многообразие существующих типов почв. Например, подзолистые почвы, типичные для северных районов нашей страны, образуются в условиях малого содержания органических остатков (гуминовых веществ) и большой влажности, вымывающей окислы основного характера (RO и R2O). Остающиеся коагели характе- ризуются высоким содержанием SiO2 и малым количеством питатель- ных веществ, необходимых для растений. Наоборот, черноземные почвы средней полосы России образуются в условиях малой влажности. В этих условиях ионы Са2+ и Mg2+ не вы- мываются и, взаимодействия с гуминовыми кислотами, образуют нерас- творимые высокомолекулярные коллоидные частицы – гуматы Са2+ и Mg2+. В процессе взаимной коагуляции положительно заряженных час- тиц R2O3 с отрицательно заряженными гуматами и SiO2 возникают
Goal of the work: Synthesis of iron hydroxide hydrosol by condensation method; determining the threshold for electrolyte coagulation of a sol and studying its dependence on the charge of the coagulating ion; determination of the protective number of a stabilizer (high molecular weight compound). (The work lasts 3 hours)
Brief theoretical introduction
Iron hydroxide hydrosol is synthesized by the condensation method by carrying out the hydrolysis reaction of ferric chloride at 100ºC:
The hydrolysis reaction of FeCl 3 proceeds intensively with the formation of highly dispersed water-insoluble Fe (OH) 3 particles.
The aggregative stability of iron hydroxide sol is ensured, first of all, by the presence of double electrical layers on the surface of dispersed particles. The elementary particle of such a sol is called a micelle. The micelle is based on an aggregate that is insoluble in a given dispersion medium and consists of many molecules (atoms): n, where n is the number of molecules (atoms) included in the aggregate.
The surface of the aggregate can be charged due to the selective adsorption of ions from the dispersion medium or the dissociation of molecules in the surface layer of the aggregate. In accordance with the Peskov-Fajans rule, ions that are part of the aggregate or that specifically interact with it are adsorbed predominantly. The ions that impart a surface charge to the aggregate are called potential-determining. The charged aggregate forms the core of the micelle.
With this method of obtaining iron hydroxide sol, the n·m Fe 3+ core has a positive surface charge due to the adsorption of Fe 3+ ions from the medium (m is the number of adsorbed ions). The charge of the nucleus is compensated by the equivalent charge of oppositely charged ions - counterions located in the volume of the medium.
Counterions located directly at the surface of the nucleus (at distances close to the diameters of the ions), in addition to electrostatic forces, experience forces of adsorption attraction of the surface. Therefore, they are especially tightly bound to the micelle core and are called counterions of the adsorption layer (their number is m - x). The remaining counterions make up a diffusely constructed ionic shell and are called counterions of the diffuse layer (their number corresponds to x).
The hydrophobic sol micelle is electrically neutral. The formula of a micelle of an ion-stabilized iron hydroxide sol can be written as follows:
aggregate potential - counterions ions diffuse
defining dense layer
ions layer
_______________________
micelle core
_________________________________________
colloidal particle
______________________________________________________
In the micelle formula, the boundaries of the colloidal particle are indicated by curly brackets. Adsorption layer thickness δ small (< 1 нм) и постоянна. Толщина диффузного слоя λ significantly larger (can be > 10 nm) and strongly depends on the concentration of electrolytes in the system.
According to the Gouy-Chapman theory, the counterions of the diffuse part of the EDL are distributed in the surface potential field in accordance with Boltzmann's law. Theory shows that the potential in the diffuse part of the layer decreases exponentially with distance. At a small potential, this dependence is expressed by the equation
φ = φ δ e – χ x(1)
Where φ δ – potential of the diffuse layer; X– distance from the beginning of the diffuse part of the DES; χ is the reciprocal of the thickness of the diffuse part of the layer.
The thickness of the diffuse part of the layer is taken to be the distance at which the potential of the diffuse part of the layer φ δ decreases by e times.
In accordance with the same theory, the thickness of the diffuse part of the layer is equal to:
Where ε 0 - electrical constant; ε - relative dielectric constant of the medium; F– Faraday constant; I– ionic strength of the solution; c 0 i– ion concentration in solution; z i– charge of the electrolyte ion.
It follows from the equation that λ decreases with increasing concentration of the electrolyte and the charge of its ions and with decreasing temperature.
When one phase moves relative to another on the sliding plane, the EDL breaks (as a rule, in the diffuse part) and the appearance of electrokinetic (“zeta”) ζ – potential (see Fig. 1).
In the process of coagulation of a highly dispersed layer of iron hydroxide, relatively small-sized sedimentation-resistant aggregates are formed.
ghats. Therefore, it is most convenient to study the coagulation of Fe(OH) 3 particles using the turbidimetric method. The applicability of this method is based on the strong dependence of the light scattering intensity on the particle size. When particles coagulate, it increases, and the optical density of the sol increases accordingly. Since when a light flux passes through colored sols, part of the light is scattered and part is absorbed, when studying coagulation in such systems by turbidimetry it is necessary to exclude the absorption of light. For Fe (OH) 3 sol, this can be achieved by taking measurements with a red filter, i.e. at the wavelength of incident light λ = 620 – 625 nm.
The threshold for rapid coagulation is determined by the threshold volume of electrolyte V to(ml), at which the optical density of the sol reaches its maximum value, and does not change with further addition of the electrolyte. The value of c k is calculated using the formula:
Where from to– concentration of the introduced electrolyte, mol/l; V– volume of sol, ml.
To prevent the aggregation of particles and protect hydrosols from the coagulating effect of electrolytes, high-molecular compounds and colloidal surfactants that are soluble in water, such as proteins, soaps, starch, and dextrin, are used. Their stabilizing effect is based on the formation of adsorption gel-like films on the surface of particles of the dispersed phase and is associated both with a decrease in interfacial tension and with the structural and mechanical properties of the surface layers.
The protective ability of polymers or surfactants relative to the selected sol is characterized by the protective number S– the amount of substance required to stabilize a unit volume of the sol. Security number S, as well as the coagulation threshold from to, determined by turbidimetry. Security number S(g/l sol) is calculated using the equation:
Where with st– concentration of stabilizer solution, g/l; V def– volume of stabilizer solution required to prevent coagulation of the sol, ml.
When coagulating with electrolytes according to the concentration mechanism (for highly charged particles), the coagulation threshold c to is inversely proportional to the charge z coagulating ion to the sixth power, i.e.
Figure 2. Dependence of optical density D sol from the volume of electrolyte - coagulator V el.
Figure 3. Dependence of optical density D sol from the volume of stabilizer solution V st.
Meaning V def corresponds to the volume of stabilizer in the ash containing the threshold volume V to electrolyte, at which on the dependence curve D= f(V st) a lower horizontal section appears (Fig. 3).
Instruments and measurement methods
Photoelectric colorimeter type FEK – 56M
Electric stove
250 ml conical flask
20 ml tubes
25 ml burettes and graduated pipettes
2% (wt.) sodium sulfate solution
0.5 M sodium acetate solution
0.01% (wt.) gelatin solution
To obtain Fe (OH) 3 hydrosol, 10 ml of ferric chloride solution is poured into a flask with 250 ml of boiling distilled water. The resulting sol, red-brown in color, is cooled to room temperature.
10 ml of sol, water and electrolyte (Na 2 SO 4 or CH 3 COONa solution) are poured into 10 test tubes in the following volumes:
Tube number... 1 2 3 4 5 6 7 8 9 10
Volume of water, ml...... 10.0 9.0 8.5 8.0 7.5 7.0 6.5 6.0 5.5 5.0
Electrolyte volume
V el, ml……………. 0 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0
The electrolyte is introduced into each sol sample 2-4 minutes immediately before measuring its optical density.
The optical density of the sol in each flask is measured using a photoelectric colorimeter using a light filter No. 8 or No. 9.
Work sequence
The obtained data is recorded in table 1.
Table 1 . Results of the study of coagulation of iron hydroxide sol by optical method.
Concerning the technology of many dosage forms.
Rule wording:
Explanation of the rule
The drug particles have cracks (Griffith's fissures) into which liquid penetrates. The liquid exerts disjoining pressure on the particle, which exceeds the contracting forces, which promotes grinding. If the substance being ground is swelling, then it is thoroughly ground in dry form and only then liquid is added. After grinding the medicinal substance, agitation is used to fractionate the particles. Rustening consists of the fact that when a solid substance is mixed with a liquid 10-20 times larger in volume than its mass, small particles are suspended, and large ones settle to the bottom. This effect is explained by different rates of sedimentation of particles of different sizes (Stokes' law). The suspension of the most crushed particles is drained, and the sediment is re-crushed and stirred with a new portion of liquid until the entire sediment turns into a thin suspension.
Application in technology
Bismuthi subnitratis ana 3.0
Aqua destillatae 200 ml
M.D.S. Wipe your face
Recipe meaning: 200 ml of purified water is measured into the stand. In a mortar, grind 3 g of starch and 3 g of basic bismuth nitrate with 3 ml of water (according to Deryagin’s rule), then add 60-90 ml of water, stir the mixture and leave for several minutes. Carefully pour the thin suspension from the sediment into a bottle. The wet sediment is additionally ground with a pestle, mixed with a new portion of water, and drained. Grinding and agitation are repeated until all large particles turn into a thin suspension.
Write a review about the article "Deryagin's Rule"
Notes
Excerpt characterizing Deryagin's Rule
She led him into the dark living room and Pierre was glad that no one there saw his face. Anna Mikhailovna left him, and when she returned, he, with his hand under his head, was fast asleep.The next morning Anna Mikhailovna said to Pierre:
- Oui, mon cher, c"est une grande perte pour nous tous. Je ne parle pas de vous. Mais Dieu vous soutndra, vous etes jeune et vous voila a la tete d"une immense fortune, je l"espere. Le testament n"a pas ete encore ouvert. Je vous connais assez pour savoir que cela ne vous tourienera pas la tete, mais cela vous impose des devoirs, et il faut etre homme. [Yes, my friend, this is a great loss for all of us, not to mention you. But God will support you, you are young, and now you are, I hope, the owner of enormous wealth. The will has not yet been opened. I know you well enough and I am sure that this will not turn your head; but this imposes responsibilities on you; and you have to be a man.]
Pierre was silent.
– Peut etre plus tard je vous dirai, mon cher, que si je n"avais pas ete la, Dieu sait ce qui serait arrive. Vous savez, mon oncle avant hier encore me promettait de ne pas oublier Boris. Mais il n"a pas eu le temps. J "espere, mon cher ami, que vous remplirez le desir de votre pere. [Afterwards, perhaps I will tell you that if I had not been there, God knows what would have happened. You know that the uncle of the third day He promised me not to forget Boris, but he didn’t have time. I hope, my friend, you will fulfill your father’s wish.]
Pierre, not understanding anything and silently, blushing shyly, looked at Princess Anna Mikhailovna. After talking with Pierre, Anna Mikhailovna went to the Rostovs and went to bed. Waking up in the morning, she told the Rostovs and all her friends the details of the death of Count Bezukhy. She said that the count died the way she wanted to die, that his end was not only touching, but also edifying; The last meeting between father and son was so touching that she could not remember him without tears, and that she does not know who behaved better in these terrible moments: the father, who remembered everything and everyone in such a way in the last minutes and such Touching words were spoken to his son, or Pierre, whom it was a pity to see how he was killed and how, despite this, he tried to hide his sadness so as not to upset his dying father. “C"est penible, mais cela fait du bien; ca eleve l"ame de voir des hommes, comme le vieux comte et son digne fils,” [It’s hard, but it’s saving; the soul rises when you see people like the old count and his worthy son,” she said. She also spoke about the actions of the princess and Prince Vasily, not approving of them, but in great secrecy and in a whisper.
The calculated ratio is compared with the ratio of rapid coagulation thresholds, which follows from the Deryagin-Landau rule (Schulze-Hardy rule).
A quantitative clarification and theoretical justification of the Schulze-Hardy rule were given by Deryagin and Landau. To calculate the coagulation threshold, the theory gives the following formula
The coagulating ability of an electrolyte is characterized by a coagulation threshold, i.e., the minimum concentration of electrolyte in a colloidal solution that causes its coagulation. The coagulation threshold depends on the valence of the coagulating ion. This dependence is expressed by the rule of significance (Schulze-Hardy rule). A more strict, theoretically substantiated quantitative relationship between the rapid coagulation threshold y and the valence of the ion is expressed by the Deryagin-Landau rule
This result, first obtained theoretically by Deryagin and Landau, refines the Schulze-Hardy rule.
Basic principles of coagulation under the influence of electrolytes. The change in the stability of sols with changes in the content of electrolytes in them was already known to the first researchers of colloidal systems (F. Selmi, T. Graham, M. Faraday, G. I. Borschov). Subsequently, thanks to the work of G. Schultz, W. Hardy, G. Picton, O. Linder, G. Freundlich, W. Pauli, G. Kreut, N. P. Peskov, A. V. Dumansky and others, extensive experimental material was accumulated and basic theoretical generalizations were made. A huge contribution to the development of the theory of electrolyte coagulation was made by Soviet scientists B.V. Deryagin et al., P.A. Rebinder and his school. Experimentally established patterns during coagulation with electrolytes are known as coagulation rules
Graph the dependence of the optical density of O on the concentration of the electrolyte Se (Fig. III.5). From the intersection point of the continuation of both straight sections of the curve, a perpendicular is lowered onto the abscissa axis and the rapid coagulation threshold is found for each electrolyte. By dividing the obtained values of coagulation thresholds by the smallest of them, a significance rule is derived and compared with the Deryagin-Landau rule.
The existence of a sharp jump in properties at a certain distance from the substrate was discovered earlier by V.V. Karasev and B.V. Deryagin when measuring the dependence of the viscosity of some organic liquids on the distance to the solid wall. All this gives the right to call such layers a special, boundary phase, since the presence of a sharp interface is the main definition of the phase. The difference with ordinary phases is that the thickness of the boundary phase is a completely definite value for a given temperature.
The Deryagin-Verwey-Overbeck theory establishes that Sk is inversely proportional to the sixth power of the valence of the coagulating ion. The same dependence is reflected by the experimentally found Schulze-Hardy rule. The obtained excellent agreement well confirms the correctness of the theory of coagulation of lyophobic sols.
Numerous objects have shown that the coagulation threshold is inversely proportional to the valence of the coagulating ions to the power of 5 to 9, often to the power of 6. Lower values of the exponent (2-3) have also been observed. Thus, the Schulze-Hardy rule assumes only a high degree of dependence of the coagulation threshold on the valence (g) of counterions. Nevertheless, it is sometimes identified with the theoretically derived Deryagin-Landau law 2.
The influence of the valence of coagulating ions on the coagulation threshold is determined by the Schulze-Hardy rule: the greater the valence of coagulating ions, the greater their coagulating force or the lower the coagulation threshold. The theoretical justification for this rule was given in 1945 by B.V. Deryagin and L.D. Landau. The relationship they found between the coagulation threshold and the valence of coagulating ions is expressed in the form
If we take into account that in the case of a barrier mechanism at r
To obtain thinner and more stable aqueous suspensions of hydrophilic swelling substances (basic bismuth nitrate, zinc oxide, magnesium oxide, calcium phosphate, carbonate and glycerophosphate, koalin, sodium bicarbonate, iron glycerophosphate), it is most advisable to use the stirring method, which is a type of dispersion method. The essence of the technique is that the substance is dispersed first in dry form, then taking into account Deryagin’s rule. The resulting thin pulp is diluted approximately 10 times with water (solution), ground and the top layer of suspension is poured into a bottle for dispensing. The agitation operation is repeated until all the substance is dispersed and obtained in the form of a fine suspension.
The influence of a lubricant on friction parameters under boundary lubrication conditions is assessed, as a rule, by the amount of oil (medium) adsorption and by its chemical activity. Adsorption capacity is taken into account mainly for the case of using a chemically inactive lubricant medium. Thus, B.V. Deryagin proposed to evaluate the effectiveness of the oil film according to the oiliness criterion, which is the ratio of the roughness of lubricated and unlubricated surfaces. Another lubricity criterion is characterized by the ratio of the difference in the work done by the friction forces of non-lubricated and lubricated surfaces during the time required to abrade a film of thickness /g to the thickness of this film. Oiliness criteria are mainly determined by the duration of residence of oil (lubricant) molecules on the friction surface and the activity of the lubricant.
In electrolyte coagulation according to the concentration mechanism (for highly charged particles), the coagulation threshold C in accordance with the Deryagin-Landau rule (the rationale for the empirical Schulze-Hardy rule) is inversely proportional to the charge of 2 counterion13 to the sixth power, i.e.
The theory of the electric double layer was developed in the works of Frumkin and Deryagin. According to their ideas, the inner layer of ions of the double electric layer, called potential-forming, is closely adjacent to a certain part of oppositely charged ions (Fig. 50, a), called opposite ions and. This part of the counterions moves with the particle and forms a 6″ thick layer called adsorption. In Fig. 50, and the boundary between such a particle and the medium is indicated by a dotted line. The remaining counterions are located in a dispersion medium, where they are distributed, as a rule, diffusely.
However, recently experimental data have been obtained that indicate the inapplicability in some cases of the Schulze-Hardy rule in the form of the Deryagin-Landau law. In experience, significant deviations from this pattern are often observed, namely, in a number of cases, the coagulating effect of electrolytes is proportional to the valence of counterions to a degree less six. According to I. F. Efremov and O. G. Usyarov, this is a deviation from
The applicability of the Deryagin theory and the Schulze-Hardy rule for the coagulation of high-molecular compounds was shown using the example of rubber latexes when they interact with electrolytes of different valences (Voyutsky, Neumann, Sandomirsky).
However, even in the first approximation considered, the theory gives good agreement with experimental data (for example, Schenkel and Kitchener data obtained on monodisperse latexes), but perhaps its most important achievement is the substantiation of the Schulze-Hardy rule, which is rightly considered the cornerstone for testing stability theories. Let's consider this explanation. An analysis of the conditions for the stability of dispersed systems shows that the boundary conditions for rapid coagulation in terms of Deryagin’s theory can be written as Utyakh = O and dOmax/ek = 0, where C/max is the maximum energy (Fig. XIII. 7). These conditions express a decrease in the barrier height to zero.
In the simplest case, q = onst. Coef. Resting temperature is, as a rule, greater than the coefficient. kinematic T., so that the starting force (starting torque) is greater than the resistance to uniform movement. More precisely physical. processes during dry T. are reflected by the so-called. according to Deryagin's two-part friction law q = F/(N + PgS), where / is added to N by the pressure caused by intermolecular forces. interaction rubbing bodies, and S-pov-et factual. contact of rubbing bodies due to the waviness and roughness of T surfaces. contact of bodies is not complete.
In works of 1937 and 1940. Deryagin, using Fuchs' formulas for the coagulation rate of interacting particles, derived a criterion for the aggregative stability of weakly charged colloidal particles for two limiting cases when the radius of the particles is much less than the thickness of the ionic atmospheres, or, in other words, the characteristic Debye length, and when the radius of the particles is much greater than the thickness of the ionic atmospheres . In the second case, the criterion generalizes and quantitatively refines the empirical Eulers-Korff rule, which is in agreement with a number of experimental facts. At the same time, the existence of a distant minimum was shown on the curve expressing the dependence of the force of interaction (repulsion) on distance.
A well-known difficulty for the theory is that the inverse sixth degree rule (the Hardy-Schulze rule refined by Deryagin and Landau) is also observed when the dimensionless potential of the surface is not only small, but less than unity. This is possible, as Glazman et al. showed. , if the product of the potential and the charge of the counterion changes little when the latter changes. A quantitative explanation for this based on the charge independence of counterion adsorption was given by Usyarov.
The most developed theory of the stability of ion-stabilized colloidal solutions has led to a number of fundamental results. The theory of highly charged sols, which considers only concentration coagulation, made it possible to substantiate the Schulze-Hardy rule in the form of Deryagin-Laidau law 2. At moderate potentials of colloidal particles, coagulation thresholds change with the valence of counterions according to the law 2, where 2 a 6, which is also in accordance. with the Schulze-Hardy rule. The theory made it possible to substantiate the various patterns of the coagulating action of mixtures of electrolytes and the effect of synergism, which could not find any explanation. It should also be noted that, based on the theory, the widespread illegality of
Having obtained the exact coagulation threshold values for all electrolytes, a significance rule is derived, for which the found threshold values are divided by the lowest coagulation threshold (for AI I3). The experimental ratio of coagulation thresholds is compared with the theoretical one, calculated according to the Deryagin-Landau rule, according to which Y a b Vai u 11 1. The results of the comparison are analyzed and the work is documented in a laboratory journal.
See pages where the term is mentioned Deryagin's rule: Synthetic polymers in printing (1961) - [p.130]
Chemistry and chemical technology
Deryagin Landau's theory of coagulation
The Deryagin-Landau rule, derived by the authors on the basis of the concepts of the physical theory of coagulation, makes it possible to determine the value of the rapid coagulation threshold, which corresponds to the disappearance of the energy barrier on the curve of the general interaction of colloidal particles depending on the distance between them. The coagulation threshold values calculated using this rule do not always coincide with the experimental values due to the fact that the coagulating effect of ions depends not only on the valence, but also on specific adsorption, which is not taken into account by the above equation.
A brilliant confirmation of the DLFO theory was the calculation by B.V. Deryagin and L.D. Landau (1941) of the relationship between the values of coagulation thresholds for electrolytes containing ions of different charge values. It turned out that the coagulation threshold is inversely proportional to the sixth degree of charge of the coagulating cone. Therefore, the values of the coagulation thresholds for one-, two-, three- and four-charged ions should be related as
This is the essence of the theory of electrical stabilization and coagulation of dispersed systems by Deryagin, Landau, Verwey and Overbeck (DLVO theory).
The coagulation of emulsions has been poorly studied experimentally, since until recently there were no reliable methods for studying this process. But the theory of coagulation of dispersed systems has been developed in detail. This is the so-called DLFO (Deryagin-Landau-Verwey-Overbeck) theory.
Let us show that in the case of a generally accepted understanding of the driving force of coagulation (aggregation), conditions (1.266) are conditions for spontaneous coagulation and determine the stability threshold in concentration and represent a generalization of the stability theory of Deryagin and Landau.
Theoretical ideas about the reasons determining the stability of lyophobic sols were further developed in the works of B.V. Deryagin and L.D. Landau. According to Deryagin’s theoretical views and experimental data, a liquid film enclosed between two solid bodies immersed in it exerts disjoining pressure on them and thereby prevents their approach. The action increases rapidly with thinning of the film and is greatly reduced by the presence of electrolytes. From this point of view, the coagulation of particles is prevented by the wedging effect of the films separating them. The introduction of electrolytes into the sol leads to a change in the electrical double layer, compression of its diffuse part and a change in the strength of the films separating the particles and, thus, to a violation of the stability of the sol. The well-developed mathematical theory of stability and coagulation by Deryagin and Landau leads to a strict physical justification of the Schulze-Hardy valence rule and at the same time provides a physical basis for the empirical patterns discovered by Ostwald.
Along with the qualitative relationships between coagulation interaction and coagulation effects, a quantitative connection is also noted between them. For sols and suspensions, the coagulation threshold is always higher than the minimum electrolyte concentration that causes a coagulation interaction detected by rheological methods. As is known, the Deryagin-Landau theory gives the following expression for the coagulation threshold
The description of the stability of lyophobic sols includes a detailed consideration of the theory of the kinetics of rapid coagulation according to Smoluchowski, an approximate presentation of the theory of stability and coagulation with electrolytes of Deryagin-Landau-Verwey-Overbeck. When describing the structure of foams, special attention is paid to the role of black films formed at certain, critical concentrations of surfactants. Here, Bulgarian scientists also play a leading role.
According to the theory of coagulation by B.V. Deryagin and L.D. Landau, during Brownian motion, colloidal particles freely approach each other at a distance of up to 10 cm (on average), however, their further approach is prevented by the so-called disjoining pressure that arises in thin layers of water located between two surfaces. Disjoining pressure is the excess (compared to hydrostatic) pressure acting from the side of a thin layer on the bounding surfaces. In sols, it is caused mainly by the mutual repulsion of counterions of the diffuse layer of approaching particles and, in addition, by the forces of molecular interaction between the surfaces of these particles and water molecules. Under the influence of electrostatic fields,
As already noted, in accordance with the Deryagin-Landau coagulation theory, a value of R0 10 m corresponds to the fixation of particles at a distance of close coagulation (strong coagulation contacts) m determines the position of particles at a distance
For the first time, a qualitative approach to studying the stability of sols was outlined by Kalman and Willstetter in 1932. The first quantitative calculations were made by B.V. Deryagin in the late 30s and then completed in the work of B.V. Deryagin and L.D. Landau (1941 .). A similar approach to studying the stability of colloidal systems was later developed in the works of Dutch researchers Verwey and Overbeck. Based on the initial letters of the main authors of the emerging physical theory of coagulation, this theory is now often called the DLFO theory.
According to the theory of coagulation by B.V. Deryagin and L.D. Landau, during Brownian motion, colloidal particles freely approach each other at a distance of up to 10 cm (on average), but their further approach is prevented by the so-called disjoining pressure,
For the first time, an explanation of the aggregative stability of dispersed systems and their coagulation with quantitative consideration of the total energy of interaction of particles was given by Deryagin, and then in more detail by Deryagin and Landau. Somewhat later, the same approach to the problems of stability and coagulation was carried out by Verwey and Overbeck. Therefore, the theory of interaction and coagulation of dispersed particles is called the Deryagin-Landau-Verwey-Overbeck theory, or DLFO for short.
It is not our task to discuss the numerous theories of coagulation developed by various researchers at the end of the last century - the beginning of this one. They are of historical interest only. Currently, the generally accepted physical theory of coagulation of lyophobic sols is Deryagin - Landau - Verwey - Overbeck, in which the degree of stability of the system is determined from the balance of molecular and electrostatic forces (see Chapter I). Although the detailed development of this theory has not yet been completed, it, thanks to a fundamentally correct interpretation of the role of surface forces of different natures, has made it possible to explain a number of colloid-chemical phenomena.
The development of a quantitative theory of stability and coagulation of colloidal systems, in particular, the DLFO theory (Deryagin-Landau-Verwey-Overbeck theory), has led, since the Second World War, to an increase in the number of studies of various colloidal systems.
N.P. Peskov found out the reason for the stability of colloidal solutions, and B. Deryagin and L. Landau developed the modern theory of coagulation. In the field of general theory of solutions, the works of N. A. Izmailov, devoted to the differentiating effect of solvents, are of great importance for analytical chemistry. In them, he used the long-known influence of the solvent on the strength of acids and bases, established that there are solvents in which this influence is especially manifested, specific to acids of different classes, i.e. it is differentiating, and using a large experimental material showed how use this phenomenon in analytical chemistry.
Thus, the theory of Deryagin and Landau is broader than the theory of coagulation. It is a theory of stabilization of colloidal systems, from which the coagulation of colloids is also derived.
The coagulation process in emulsions is described by the DLVO (Deryagin-Landau-Verwey-Overbeck) theory. Its essence boils down to the fact that in the presence of hydrophilic areas on the globules of the dispersed phase and the particles approaching at a distance of action of dispersion forces, they aggregate into conglomerates of particles of progressively increasing size. This process occurs with a decrease in free energy and occurs spontaneously. The presence of a structural-mechanical barrier around the dispersed phase globules does not protect them from adhesion to the outer layers, although it depends on the viscosity of the external environment. The rate of coagulation in a concentrated system can be estimated from the kinetics of increase in its structural and mechanical properties, if the rate of coalescence of globules is small compared to the rate of their coagulation.
Aggregative stability and long-term existence of lyophobic D.s. with the preservation of their properties is ensured by stabilization. For highly dispersed systems with a liquid dispersion medium, the introduction of stabilizers (electrolytes, surfactants, polymers) is used. In the Deryagin-Landau-Verwey-Overbeck stability theory (DLFO theory) basic. the role is played by ion-electrostatic. stabilization factor. Stabilization is provided electrostatically. repulsion of diffuse parts of double electric. layer, which is formed by the adsorption of electrolyte ions on the surface of particles. At a certain distance between particles, the repulsion of diffuse layers determines the presence of a minimum potential. curve (far, or secondary, minimum, see figure). Although this minimum is relatively shallow, it can prevent further convergence of particles attracted by the forces of intermolecular interaction. The near, or primary, minimum corresponds to strong adhesion of particles, in which case the energy of thermal motion is not enough to separate them. When approaching a distance corresponding to this minimum, the particles combine into aggregates, the formation of which leads to the loss of aggregative stability by the system. In this case, the stability of the system to coagulation is determined by the height of the energy. barrier.
The main scientific works are devoted to the study of surface phenomena. He developed the thermodynamics of systems taking into account the concept of disjoining pressure of thin layers that he introduced. For the first time, he carried out direct measurements of the molecular attraction of solids as a function of distance and disjoining pressure of thin layers of liquids. He theoretically substantiated the influence of the overlap of ionic atmospheres on the disjoining pressure of liquid layers and the interaction of colloidal particles, which allowed him to create the theory of coagulation and heterocoagulation of colloidal and dispersed systems. Together with the Soviet physicist L.D. Landau created (1928) the theory of stability of lyophobic colloids, now known as the DLFO theory (theory of stability of dispersed systems of Deryagin - Landau - Verwey - Overbeck). He discovered special properties of boundary layers of liquids, determined by their specific (anisotropic) structure. He developed the theories of thermoosmosis and capillary osmosis in liquids, thermophoresis and diffusionophoresis of aerosol particles. Author of the two-term law of external friction. Under his leadership, whisker-like diamond crystals were synthesized for the first time at low pressures. He developed methods for growing diamond crystals and powders from gas at low pressures.
The applicability of the Deryagin-Landau-Verwey-Overbeck theory for describing the stability and coagulation of dispersions in non-polar media was substantiated by Parfit et al. , who carefully analyzed the factors that complicate the quantitative description of coagulation processes.
Important P. I. - surface activity, manifested in a decrease in surface tension during the adsorption of one of the components of the solution. Surfactants have a huge practical effect. significance as regulators of P. i. they affect wetting, dispersion, adhesion, etc. The role of surfactants is especially important in colloidal systems that have a large excess of surface energy. Thermodynamic instability of such systems. manifests itself in coagulation and coalescence/gnosis when particles approach each other, which can be hampered by disjoining pressure resulting from the overlap of the surface layers of approaching particles. On this basis, physical science arose. theory of stability of colloids Deryagin - Landau - Verwey - Overbeck.
The most developed theory of the stability of ion-stabilized colloidal solutions has led to a number of fundamental results. The theory of highly charged sols, which considers only concentration coagulation, made it possible to substantiate the Schulze-Hardy rule in the form of Deryagin-Landau law 2. At moderate potentials of colloidal particles, coagulation thresholds change with the valence of counterions according to the law 2, where 2 a See pages where the term is mentioned Deryagin Landau's theory of coagulation: Adhesion of liquid and wetting (1974) - [p.196]
Landau-Deryagin rule
History of the development of colloid chemistry
Let's get to know each other better
Coagulation rules
1. All strong electrolytes added to the sol in sufficient quantities cause its coagulation.
The minimum concentration of electrolyte that causes coagulation of the sol in a certain short period of time is called coagulation threshold.
The coagulation threshold can be calculated by knowing the concentration of the coagulating electrolyte C, the volume of added electrolyte V, and the volume of sol V of the sol (usually 10 ml): The reciprocal of the coagulation threshold is called coagulating ability electrolyte. This means that the lower the coagulation threshold, the greater the coagulating ability of the electrolyte.
2. Not the entire electrolyte has a coagulating effect, but only that ion whose charge coincides in sign with the charge of the counterions of the micelle of the lyophobic sol (the charge of the coagulating ion is opposite to the charge of the colloidal particle). This ion is called ion - coagulant.
3. The greater the charge of the ion, the greater the coagulating ability of the coagulant ion. This pattern is quantitatively described by empirical Schulze–Hardy rule, and a theoretically substantiated relationship between the charge of the coagulating ion and the coagulation threshold is given by Deryagin–Landau theory.
The ratio of coagulation thresholds for one-, two- and trivalent ions is equal to ( value rule) :
Consequently, the coagulating ability of a triply charged ion is 729 times higher than the coagulating ability of a singly charged ion.
Currently, deviations from the Schulze–Hardy–Deryagin–Landau rule (rule of significance) have been established. In addition to the charge, the coagulation threshold is influenced by the radius of the coagulating ion, the ability for adsorption and hydration, as well as the nature of the ion accompanying the coagulating one.
When multi-charged ions, such an effect as particle recharging, i.e. change in the sign of charge and potential of a colloidal particle. Added ions can exchange with counterions, replacing them in both the diffuse and adsorption layers. Moreover, if the multiply charged ion is small enough (for example, Al 3+, Th 4+, etc.), it replaces on the surface of the particles (in the adsorption layer) non-equivalent in charge number of former ions ( superequivalent adsorption). For example, instead of one or two K + ions, there may be a Th 4+ ion. Therefore, at a sufficiently high concentration of such ions, the charge they create on the surface can become greater in absolute value than the charge of potential-determining ions. This means a change in the sign of charge and potential. Now such ions become potential-determining (instead of the previous ones) and other counterions are oriented around the particle.
4. The coagulating ability of an ion with the same charge is greater, the greater greater is its crystal radius.
For singly charged inorganic cations, the coagulating ability decreases in the following order:
Ag + > Cs + > Rb + > NH 4 + > K + > Na + > Li +
sites.google.com
Rules for coagulation with electrolytes
Coagulation is observed when a certain amount of any electrolyte is added that does not chemically react with the dispersed phase of the system. Observations by G. Schulze established that coagulation is caused by one of the electrolyte ions. This ion is called the coagulating ion. Moreover, the coagulating ability of the ion increases with increasing charge of the ion in geometric progression at a ratio of 1:100:1000 (rule of significance or Schulze’s rule). Landau, Deryagin established that the coagulating ability changes in accordance with the 6th degree of charge of the ions: 1 6:2 6:3 6 = 1:64:729.
The patterns found by Schulze and Hardy are combined into one rule (Schulze-Hardy rule): the coagulating effect is that of the electrolyte ion, the charge of which is opposite to the charge of the granule and the coagulating effect is stronger, the higher the charge of the coagulating ion.
 , mol/l.
, mol/l.
The coagulation threshold depends on a number of conditions: from the moment of fixation after adding the electrolyte; from the method of observation; on the concentration of the test solution and the added electrolyte. The coagulation threshold is determined by measuring light scattering or titrating a colloidal solution with an electrolyte until obvious coagulation begins.
The reciprocal of the coagulation threshold is called coagulating ability: . It expresses the volume of sol coagulated under the action of 1 mmol of a coagulating ion. The higher the coagulating ability, the less electrolyte is available to induce coagulation.
Coagulating ability depends on atomic mass and charge, i.e. ion charge density. As the atomic mass increases, the charge density decreases and the ions become less polarized. As a result, their solvation shell becomes thinner. Therefore, large ions penetrate more easily into the adsorption layer of the micelle and neutralize the charge of the particle, causing coagulation of the sol. For example, for a silver iodide sol of composition xK +, the indifferent electrolytes are KNO 3, NaNO 3, Ca(NO 3) 2, Al(NO 3) 3, Th(NO 3) 4, and the coagulating ions are K +, Na +, Ca 2+, Al 3+, Th 4+. The coagulating ability of ions increases in the series: Li + + + + + or Na + 2+ 3+ 4+. The lower the hydration (solvation) of the cation, the lower the coagulation threshold, i.e. stronger coagulating effect. The hydration shell increases the size of the ion and prevents the ion from penetrating into the adsorption layer. The coagulating ability of organic compounds increases in accordance with Traube's rule.
Later, M. Hardy discovered that the charge of the coagulating ion is always opposite to the charge of the micelle granule (Hardy's rule). Consequently, the negative granule coagulates under the influence of positively charged ions, and the positively charged granule coagulates under the influence of the anions of the added electrolyte.
To characterize and compare different electrolytes, the concept of “coagulation threshold” is used - this is the minimum concentration of the added electrolyte at which coagulation begins (is observed):
 , mol/l.
, mol/l.
The reciprocal of the coagulation threshold is called coagulating ability:  . It expresses the volume of sol coagulated under the action of 1 mmol of a coagulating ion. The higher the coagulating ability, the less electrolyte is available to induce coagulation.
. It expresses the volume of sol coagulated under the action of 1 mmol of a coagulating ion. The higher the coagulating ability, the less electrolyte is available to induce coagulation.
Theories of coagulation by electrolytes
Existing theories of coagulation have tried to answer 3 questions:
- why does coagulation occur at a certain concentration of electrolyte-coagulator?
- why does the concentration of the ion opposite to the charge of the granule play the main role?
- why does the influence of the charge of the coagulator ion obey the Schulze-Hardy rule?
Freundlich adsorption theory. According to this theory, coagulating ions on the surface of particles are adsorbed in accordance with the adsorption isotherm:  . Moreover, coagulation occurs with a gradual, equal decrease in the zeta potential due to the adsorption of an equivalent amount of different ions. Due to neutralization, the number of charges of potential-determining ions decreases, which leads to a decrease z-potential to a critical value.
. Moreover, coagulation occurs with a gradual, equal decrease in the zeta potential due to the adsorption of an equivalent amount of different ions. Due to neutralization, the number of charges of potential-determining ions decreases, which leads to a decrease z-potential to a critical value.
The limitation of the theory is that in practice equivalent adsorption is not always observed, the adsorption isotherms of different ions are different, and sometimes coagulation affects only the diffuse layer.
Muller's electrostatic theory. According to this theory, the introduction of an electrolyte does not change the total charge in the DES, but only causes compression of the diffuse layer (displacement of counterions into the adsorption layer). A decrease in the thickness of the ionic atmosphere leads to a decrease in z-potential, which reduces the stability of the sol.
This theory does not take into account the adsorption of introduced ions and their entry into the EDL.
Both theories are valid, both take place during coagulation, but at different stages. Due to limitations, they cannot be used to explain other types of coagulation.
DLFO theory developed by Deryagin, Landau, Verwey and Overbeck (1941). In accordance with the first letters of the authors' surnames, it is called DLFO. It takes into account the potential energy of particles and the equilibrium of e/static forces acting between them. When particles approach each other, e/static forces of attraction and repulsion arise between them. The state of the system is determined by their ratio. If the repulsive force is greater, then the system is stable. The predominance of attractive energy causes coagulation. The attractive energy is due to van der Waals forces and varies inversely with the square of the distance between particles:  . These forces act only at very small distances (1.10 − 10 – 1.10 − 11 m, i.e. 1/10 of the size of colloidal particles). Therefore, coagulation is observed only when particles approach each other at the proper distance. This approach occurs during the thermal movement of particles and therefore influences that increase the speed of movement of particles and the number of collisions (see factors causing coagulation) promote coagulation.
. These forces act only at very small distances (1.10 − 10 – 1.10 − 11 m, i.e. 1/10 of the size of colloidal particles). Therefore, coagulation is observed only when particles approach each other at the proper distance. This approach occurs during the thermal movement of particles and therefore influences that increase the speed of movement of particles and the number of collisions (see factors causing coagulation) promote coagulation.

Fig.1. Overlap of ionic atmospheres of colloidal particles
As the distance between particles decreases, the forces of electrostatic repulsion increase. The solvation shell also prevents the particles from coming into contact. Typically, electrostatic repulsion forces appear when diffuse layers (ionic spheres) of similarly charged particles overlap. The repulsion energy decreases with increasing distance between them.

Fig.2. Potential coagulation curve
To determine the state of the system, the total energy is calculated (a potential coagulation curve is constructed). It has several sections: a deep primary minimum (potential well 1) in the region of small distances, a shallow secondary minimum (potential well 2) in the region of large distances. They indicate a significant predominance of attractive energy, i.e. in them U pr >> U ott.
In the area of average distances there is a maximum. If it is located above the x-axis, then repulsive forces act between the particles, i.e. the system is aggregatively stable. In this case, U out >> U in. The higher the maximum, the more stable the system.
To begin coagulation, preliminary partial neutralization of the particle charge to a certain value and destruction of the solvation shell is sufficient. This is achieved by introducing an electrolyte or removing a stabilizing electrolyte. The minimum particle charge at which coagulation begins is called critical z-potential (
0.03 V). At a critical value of the zeta potential, the kinetic energy of particle motion is sufficient to overcome the forces of residual electrostatic repulsion (U pr
U ott) and the adhesion of particles into aggregates.
According to the DLFO theory, during rapid coagulation with electrolytes, two mechanisms are distinguished: concentration coagulation and adsorption (neutralization) coagulation.
At concentration coagulation added indifferent ions do not change the value of the -potential. Coagulation occurs due to compression of the diffuse layer, i.e. displacement of counterions into the adsorption layer or by increasing the ionic strength of the solution.
Adsorption coagulation occurs as a result of a decrease in -potential. This type of coagulation is caused by electrolytes, the ions of which can (are able to) be adsorbed on the surface of particles and have a charge opposite to that of the granule. Penetrating into the adsorption layer, they neutralize potential-determining ions and reduce the -potential.
If there are free centers on the surface of microcrystals, then the crystal lattice is completed. For example, in the case of x K + sol, the addition of KI causes coagulation due to the adsorption of iodide ions. In this case, first the - and -potentials increase. After the centers are saturated, adsorption stops. A further increase in the concentration of KI leads to a decrease in the -potential due to compression of the diffuse layer (displacement of potassium ions into the adsorption layer). When a certain concentration is reached, the sol begins to coagulate.

If there are no free centers on the surface, then adsorption is not observed and the -potential does not increase, but compression of the diffuse layer occurs.
When AgNO 3 is added, silver ions Ag + are non-indifferent. Since the potential-determining ions are iodide ions, the addition of silver ions leads to the formation of a sparingly soluble compound AgI. As a result, the number of potential-determining ones gradually decreases, which leads to a decrease in - and -potentials. At a critical value of the -potential, the sol coagulates according to the adsorption mechanism. Further addition of AgNO 3 leads to recharging and increasing the positive charge of the granule due to the selective adsorption of silver ions with the formation of a new DES: x NO 3 ─. With further addition of AgNO 3, the sol coagulates according to the concentration mechanism under the influence of nitrate ions.