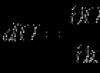Heat capacity body (usually denoted Latin letter C) - physical quantity determined by the ratio of an infinitesimal amount of heat δ Q, received by the body, to the corresponding increment of its temperature δ T :
The unit of heat capacity in the International System of Units (SI) is J/.
Specific heat
Specific heat capacity is the heat capacity per unit amount of a substance. The amount of a substance can be measured in kilograms, cubic meters and pray. Depending on which quantitative unit refers to heat capacity; distinguishes between mass, volumetric and molar heat capacity.
Mass specific heat capacity ( WITH), also simply called specific heat capacity, is the amount of heat that must be supplied to a unit mass of a substance to heat it by a unit temperature. In SI it is measured in joules per kilogram per kelvin (J kg −1 K −1).
And when constant pressure
Transfer of matter from one state of aggregation in another accompanied spasmodic a change in heat capacity at a specific temperature point of transformation for each substance - the melting point (transition of a solid into a liquid), the boiling point (transition of a liquid into a gas) and, accordingly, the temperatures of reverse transformations: freezing and condensation.
The specific heat capacities of many substances are given in reference books, usually for a process at constant pressure. For example, the specific heat capacity of liquid water at normal conditions- 4200 J/(kg K); ice - 2100 J/(kg K).
Heat capacity theory
There are several theories of the heat capacity of a solid:
- Dulong-Petit law and Joule-Copp law. Both laws are derived from classical concepts and, with a certain accuracy, are valid only for normal temperatures(approx. 15°C to 100°C).
- Einstein's quantum theory of heat capacities. First use quantum laws to the description of heat capacity.
- Debye's quantum theory of heat capacities. Contains the most full description and is in good agreement with experiment.
The heat capacity of a system of non-interacting particles (for example, ideal gas) is determined by the number of degrees of freedom of the particles.
Write a review about the article "Heat capacity"
Notes
Literature
- // Encyclopedic Dictionary young physicist/ V. A. Chuyanov (compiled). - M.: Pedagogy, 1984. - P. 268–269. - 352 s.
See also
Excerpt characterizing Heat Capacity
He could not have a goal, because he now had faith - not faith in some rules, or words, or thoughts, but faith in a living, always felt God. Previously, he sought it for the purposes that he set for himself. This search for a goal was only a search for God; and suddenly he learned in his captivity, not in words, not by reasoning, but by direct feeling, what his nanny had told him long ago: that God is here, here, everywhere. In captivity, he learned that God in Karataev is greater, infinite and incomprehensible than in the Architect of the universe recognized by the Freemasons. He experienced the feeling of a man who had found what he was looking for under his feet, while he strained his vision, looking far away from himself. All his life he had been looking somewhere, over the heads of the people around him, but he should have not strained his eyes, but only looked in front of him.He had not been able to see before the great, incomprehensible and infinite in anything. He just felt that it must be somewhere and looked for it. In everything close and understandable, he saw something limited, petty, everyday, meaningless. He armed himself with mental spotting scope and looked into the distance, to where this small, everyday thing, hiding in the fog of the distance, seemed great and endless to him only because it was not clearly visible. This is how he imagined European life, politics, Freemasonry, philosophy, philanthropy. But even then, in those moments that he considered his weakness, his mind penetrated into this distance, and there he saw the same petty, everyday, meaningless things. Now he had learned to see the great, the eternal and the infinite in everything, and therefore naturally, in order to see it, to enjoy its contemplation, he threw down the pipe into which he had been looking until now through the heads of people, and joyfully contemplated the ever-changing, ever-great world around him. , incomprehensible and endless life. And the closer he looked, the more calm and happy he was. Previously, the terrible question that destroyed all his mental structures was: why? did not exist for him now. Now to this question - why? a simple answer was always ready in his soul: because there is a God, that God, without whose will a hair will not fall from a man’s head.
Pierre has hardly changed in his external techniques. He looked exactly the same as he had been before. Just as before, he was absent-minded and seemed preoccupied not with what was in front of his eyes, but with something special of his own. The difference between his previous and present state was that before, when he forgot what was in front of him, what was said to him, he, wrinkling his forehead in pain, seemed to be trying and could not see something far away from him . Now he also forgot what was said to him and what was in front of him; but now, with a barely noticeable, seemingly mocking, smile, he peered at what was in front of him, listened to what was being said to him, although obviously he saw and heard something completely different. Before, although he seemed to be a kind person, he was unhappy; and therefore people involuntarily moved away from him. Now a smile of the joy of life constantly played around his mouth, and his eyes shone with concern for people - the question: are they as happy as he is? And people were pleased in his presence.
Before, he talked a lot, got excited when he spoke, and listened little; Now he rarely got carried away in conversation and knew how to listen so that people willingly told him their most intimate secrets.
The princess, who had never loved Pierre and had a particularly hostile feeling towards him since, after the death of the old count, she felt obliged to Pierre, to her chagrin and surprise, after a short stay in Orel, where she came with the intention of proving to Pierre that, Despite his ingratitude, she considers it her duty to follow him; the princess soon felt that she loved him. Pierre did nothing to ingratiate himself with the princess. He just looked at her with curiosity. Previously, the princess felt that in his gaze at her there was indifference and mockery, and she, as in front of other people, shrank before him and showed only her fighting side life; now, on the contrary, she felt that he seemed to be getting to the bottom of the most intimate aspects of her life; and she, at first with distrust, and then with gratitude, showed him the hidden good sides of her character.
Most cunning man He could not have more skillfully insinuated himself into the princess’s confidence, evoking her memories of the best time of her youth and showing sympathy for them. Meanwhile, Pierre’s whole cunning consisted only in the fact that he sought his own pleasure, evoking human feelings in the embittered, dry and proud princess.
- Yes, he is very, very kind person“When she is under the influence not of bad people, but of people like me,” the princess told herself.
The change that took place in Pierre was noticed in their own way by his servants, Terenty and Vaska. They found that he had slept a lot. Terenty often, having undressed the master, with boots and dress in his hand, wishing him good night, hesitated to leave, waiting to see if the master would enter into conversation. AND for the most part Pierre stopped Terenty, noticing that he wanted to talk.
Heat capacity of gas. The heat capacity of a body CT is the ratio of the amount of heat Q imparted to the body to the temperature change ∆T
The heat capacity of a body C T is the ratio the amount of heat Q imparted to the body to the temperature change ∆T caused by this heat transfer.
Distinguish specific heat capacity of a substance(c) and molar heat capacity (C).
The specific heat capacity of a substance is the amount of heat required to heat 1 kg substances per 1 TO
Molar heat capacity is the amount of heat required to heat 1 mole of a substance by 1K.
There is an obvious connection between specific and molar heat capacities
It turns out that the heat capacity depends significantly on the conditions under which the gas is heated. Distinguish heat capacity at constant volume C v and heat capacity at constant pressure C p. At constant temperature The heat capacity is equal to infinity, since ∆T= 0.
Consider 1 mole of gas heated at constant volume (V= const, v = t/μ = 1 mol). Based on the first law of thermodynamics, all heat supplied to the gas goes to change it internal energy Q = ∆U.
Let us obtain an expression for the heat capacity of gas at constant volume.
Considering that ∆U = (i/2)v/R∆T, we get:
Thus, the molar heat capacity at constant volume depends only on the number of degrees of freedom i gas molecules, i.e. on the number of atoms in the molecule and on its structure.
Now let 1 mole of gas be heated at constant pressure (p = const, v = 1 mol). In this case, the heat supplied to the gas goes, in accordance with the first law of thermodynamics, not only to change its internal energy, but also to the work of gas expansion (it is the expansion of the gas that ensures constant pressure). And this means that The heat capacity of a gas at constant pressure is greater than its heat capacity at constant volume (C p > C v ). To find their difference, let us first calculate the work of expansion of 1 mole of gas during an isobaric process. In accordance with formula (3), this work A= р∆ V= p(V 2 – V 1) = pV 3 – pV 1. Let us take into account the Mendeleev-Clapeyron equation, then
Formula (11) allows you to set physical meaning universal gas constant R. By condition p = const and v = 1 mol; let us assume that ∆T = 1K, then numerically A = R or the universal gas constant is numerically equal to the work of expansion of one mole of an ideal gas when heated to 1K at constant pressure.
To find the formula for the heat capacity of a gas at constant pressure, we use the definition of molar heat capacity (7) and the first law of thermodynamics
Taking into account formula (9) in the first term and (11) in the second, we obtain
This expression is called Mayer's equation. Let us substitute expression (10) into this equation and obtain
An important thermodynamic characteristic is the ratio of heat capacity at constant pressure to heat capacity at constant volume τ
From formulas (10) and (13) it follows that according to the values of heat capacities, all gases can be divided into three types: monoatomic, diatomic and polyatomic gases. So it's easy to count everything possible values their heat capacities. All results and conclusions from this paragraph can be attributed to classical theory heat capacities. Direct measurements showed that this theory is fully valid only for monatomic gases. Di- and polyatomic gases provide significant differences experimental values heat capacities from theoretical ones, especially at temperatures significantly different from normal. This question is considered most fully and correctly by the quantum theory of heat capacity. Expressions for heat capacity solids can be found in lecture No. 17.
The change in internal energy by doing work is characterized by the amount of work, i.e. work is a measure of the change in internal energy in this process. The change in the internal energy of a body during heat transfer is characterized by a quantity called the amount of heat.
is a change in the internal energy of a body during the process of heat transfer without performing work. The amount of heat is indicated by the letter Q .
Work, internal energy and heat are measured in the same units - joules ( J), like any type of energy.
In thermal measurements, a special unit of energy was previously used as a unit of heat quantity - the calorie ( feces), equal to the amount of heat required to heat 1 gram of water by 1 degree Celsius (more precisely, from 19.5 to 20.5 ° C). This unit, in particular, is currently used when calculating heat consumption (thermal energy) in apartment buildings. The mechanical equivalent of heat has been experimentally established - the relationship between calorie and joule: 1 cal = 4.2 J.

When a body transfers a certain amount of heat without doing work, its internal energy increases; if the body gives off a certain amount of heat, then its internal energy decreases.
If you pour 100 g of water into two identical vessels, one and 400 g into the other at the same temperature and place them on identical burners, then the water in the first vessel will boil earlier. Thus, than more mass body, so more it needs heat to warm up. It's the same with cooling.

The amount of heat required to heat a body also depends on the type of substance from which the body is made. This dependence of the amount of heat required to heat a body on the type of substance is characterized by a physical quantity called specific heat capacity substances.
- This physical quantity, equal to the amount of heat that must be imparted to 1 kg of a substance to heat it by 1 ° C (or 1 K). 1 kg of substance releases the same amount of heat when cooled by 1 °C.
Specific heat capacity is designated by the letter With. The unit of specific heat capacity is 1 J/kg °C or 1 J/kg °K.
The specific heat capacity of substances is determined experimentally. Liquids have a higher specific heat capacity than metals; Water has the highest specific heat, gold has a very small specific heat.
Since the amount of heat is equal to the change in the internal energy of the body, we can say that the specific heat capacity shows how much the internal energy changes 1 kg substance when its temperature changes by 1 °C. In particular, the internal energy of 1 kg of lead increases by 140 J when heated by 1 °C, and decreases by 140 J when cooled.
Q required to heat a body of mass m on temperature t 1 °C up to temperature t 2 °С, is equal to the product of the specific heat capacity of the substance, body mass and the difference between the final and initial temperatures, i.e.Q = c ∙ m (t 2 - t 1)
The same formula is used to calculate the amount of heat that a body gives off when cooling. Only in this case should the final temperature be subtracted from the initial temperature, i.e. from greater value subtract the lesser temperature.

This is a summary of the topic “Amount of heat. Specific heat". Select next steps:
- Go to next summary:
Material from Uncyclopedia
The heat capacity of a body is the amount of heat that must be imparted to a given body in order to increase its temperature by one degree. When the body cools by one degree, it gives off the same amount of heat. Heat capacity is proportional to body mass. The heat capacity of a unit mass of a body is called specific heat, and the product of the specific heat capacity and the atomic or molecular weight- atomic or molar, respectively.
Heat capacities various substances differ greatly from each other. Thus, the specific heat capacity of water at 20° C is 4200 J/kg K, pine wood - 1700, air - 1010. For metals it is less: aluminum - 880 J/kg K, iron - 460, copper - 385, lead - 130. The specific heat capacity increases slightly with temperature (at 90°C the heat capacity of water is 4220 J/kg K) and changes greatly during phase transformations: the heat capacity of ice at 0°C is 2 times less than that of water; The heat capacity of water vapor at 100°C is about 1500 J/kg K.
Heat capacity depends on the conditions under which changes in body temperature occur. If the size of the body does not change, then all the heat goes to change the internal energy. Here we are talking about heat capacity at constant volume (C V). At constant external pressure thanks to thermal expansion is being done mechanical work against external forces, and heating to a particular temperature requires more heat. Therefore, the heat capacity at constant pressure C P is always greater than C V . For ideal gases C P - C V = R (see figure), where R is the gas constant equal to 8.32 J/mol K.
Usually measured by C P . Classic way heat capacity measurements are as follows: the body whose heat capacity (C x) is to be measured is heated to a certain temperature t x and placed in a calorimeter with an initial temperature t 0, filled with water or other liquid with a known heat capacity (C k and C w - the heat capacity of the calorimeter and liquid) . Measuring the temperature in the calorimeter after establishing thermal equilibrium(t), you can calculate the heat capacity of the body using the formula:
C x = (t-t 0)(C f m f + C k m k) / (m x (t x -t)),
where m x, m f and m k are the masses of the body, liquid and calorimeter.
The most developed theory is the heat capacity of gases. At ordinary temperatures, heating leads mainly to a change in the energy of translational and rotational movement gas molecules. For the molar heat capacity of monatomic gases, C V theory gives 3R/2, diatomic and polyatomic gases - 5R/2 and 3R. At very low temperatures heat capacity is slightly less due to quantum effects(cm. Quantum mechanics). At high temperatures is added vibrational energy, and the heat capacity of polyatomic gases increases with increasing temperature.
The atomic heat capacity of crystals, according to classical theory, is equal to 3Ry, which is consistent with the empirical law of Dulong and Petit (established in 1819 by French scientists P. Dulong and A. Petit). Quantum theory heat capacity leads to the same conclusion at high temperatures, but predicts a decrease in heat capacity as temperature decreases. Up close absolute zero the heat capacity of all bodies tends to zero (third law of thermodynamics).
The heat capacity of a body is the amount of heat that must be imparted to a given body in order to increase its temperature by one degree. When the body cools by one degree, it gives off the same amount of heat. Heat capacity is proportional to body mass. The heat capacity of a unit mass of a body is called specific heat, and the product of specific heat capacity and atomic or molecular mass is called atomic or molar mass, respectively.
The heat capacities of different substances vary greatly. Thus, the specific heat capacity of water at 20° C is 4200 J/kg K, pine wood - 1700, air - 1010. For metals it is less: aluminum - 880 J/kg K, iron - 460, copper - 385, lead - 130. The specific heat capacity increases slightly with temperature (at 90°C the heat capacity of water is 4220 J/kg K) and changes greatly during phase transformations: the heat capacity of ice at 0°C is 2 times less than that of water; The heat capacity of water vapor at 100°C is about 1500 J/kg K.
Heat capacity depends on the conditions under which changes in body temperature occur. If the size of the body does not change, then all the heat goes to change the internal energy. This refers to heat capacity at constant volume. At constant external pressure, due to thermal expansion, mechanical work is performed against external forces, and heating to a particular temperature requires more heat. Therefore, the heat capacity at constant pressure is always greater than . For ideal gases (see figure), where R is the gas constant equal to 8.32 J/mol K.
Usually measured. The classic way to measure heat capacity is as follows: the body whose heat capacity is to be measured is heated to a certain temperature and placed in a calorimeter with an initial temperature filled with water or other liquid with a known heat capacity and - the heat capacity of the calorimeter and liquid).
By measuring the temperature in a calorimeter after thermal equilibrium has been established, the heat capacity of the body can be calculated using the formula:
![]()
where and are the masses of the body, liquid and calorimeter.
The most developed theory is the heat capacity of gases. At ordinary temperatures, heating leads mainly to a change in the energy of translational and rotational motion of gas molecules. For the molar heat capacity of monatomic gases, the theory gives , diatomic and polyatomic gases - and . At very low temperatures, the heat capacity is somewhat lower due to quantum effects (see Quantum mechanics). At high temperatures, vibrational energy is added, and the heat capacity of polyatomic gases increases with increasing temperature.
The atomic heat capacity of crystals, according to classical theory, is equal to , which is consistent with the empirical law of Dulong and Petit (established in 1819 by French scientists P. Dulong and A. Petit). The quantum theory of heat capacity leads to the same conclusion at high temperatures, but predicts a decrease in heat capacity as the temperature decreases. Near absolute zero, the heat capacity of all bodies tends to zero (third law of thermodynamics).