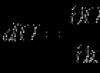| Hole | |
| Symbol: | h(English hole) |
|---|---|
 When an electron leaves a helium atom, a hole is left in its place. In this case, the atom becomes positively charged. |
|
| Compound: | Quasiparticle |
| Classification: | Light holes, heavy holes |
| Named after whom and/or what: | Lack of electron |
| Quantum0 numbers: | |
| Electric charge: | +1 |
| Spin: | Determined by the spin of electrons in the valence band ħ |
Definition according to GOST 22622-77: “Blank valence bond, which manifests itself as positive charge, numerically equal to charge electron."
Hole conduction can be explained using the following analogy: There are a number of people sitting in an auditorium where there are no spare chairs. If someone from the middle of the row wants to leave, he climbs over the back of the chair into the empty row and leaves. Here the empty row is an analogue of the conduction band, and the departed person can be compared to a free electron. Let's imagine that someone else comes and wants to sit down. It’s hard to see from an empty row, so he doesn’t sit there. Instead, the person sitting near the empty chair moves to it, and all his neighbors repeat this after him. Thus, empty space as if moving to the edge of the row. When this place is near the new viewer, he can sit down.
In this process, each person sitting moved along the row. If the spectators had a negative charge, such motion would be electrical conduction. If, in addition, the chairs are positively charged, then only free space. This simple model, showing how hole conduction works. However, in fact, due to the properties of the crystal lattice, the hole is not located in a specific place, as described above, but is spread over an area measuring many hundreds of unit cells.
Doping crystals with acceptor impurities is used to create holes in semiconductors. In addition, holes can also occur as a result external influences: thermal excitation of electrons from the valence band to the conduction band, illumination with light or irradiation with ionizing radiation.
In case Coulomb interaction holes with an electron from the conduction band are formed bound state, called an exciton.
Heavy holes- the name of one of the branches of the energy spectrum of the valence band of the crystal.
Holes in quantum chemistry
The term hole is also used in computational chemistry, where the ground state of a molecule is interpreted as a vacuum state—this state has no electrons. In such a scheme, the absence of an electron in a normally filled state is called a hole and is considered as a particle. And the presence of an electron in normally empty space is simply called an electron.
One of the most remarkable and exciting discoveries recent years was the application of physics solid to the technical development of a number of electrical devices such as transistors. The study of semiconductors led to their discovery useful properties and to many practical applications. In this area, everything is changing so quickly that what you are told today may, in a year, turn out to be incorrect or, in any case, incomplete. And it is absolutely clear that, having studied such substances in more detail, we will eventually be able to accomplish much more amazing things. You won't need the material in this chapter to understand the following chapters, but you may want to see that at least some of what you've learned is relevant in some way.
There are a lot of semiconductors known, but we will limit ourselves to those that are most used in technology today. In addition, they have been studied better than others, so that having understood them, we will, to some extent, understand many others. The most widely used semiconductor substances currently are silicon and germanium. These elements crystallize in a diamond-type lattice, a cubic structure in which the atoms have a quadruple (tetrahedral) bond with their nearest neighbors. At very low temperatures(close absolute zero) they are insulators, although they conduct little electricity at room temperature. These are not metals; they are called semiconductors.
If we somehow introduce an additional electron into a silicon or germanium crystal at a low temperature, then what is described in previous chapter. Such an electron will begin to wander around the crystal, jumping from the place where one atom stands to the place where another stands. We have only considered the behavior of an atom in a rectangular lattice, and for a real lattice of silicon or germanium the equations would be different. But everything essential can become clear from the results for a rectangular lattice.
As we saw in Chap. 11, these electrons’ energies can only be in a certain range of values, called conduction zone. In this zone, the energy is related to the wave number k of the probability amplitude WITH[cm. (11.24)] by the formula
Different A are the amplitudes of jumps in the directions x, y And z, A a, b, c - these are the lattice constants (intervals between nodes) in these directions.
For energies near the bottom of the zone, formula (12.1) can be approximately written as follows:
(see Chapter 11, § 4).
If we are interested in the movement of an electron in some specific direction, so that the ratio of the components k is the same all the time, then the energy is quadratic function wave number and, therefore, electron momentum. You can write
 where α is some constant, and draw a graph of the dependence E from k(Fig. 12.1). We will call such a graph an “energy diagram.” An electron in a certain state of energy and momentum can be represented on such a graph by a point (S
in the figure).
where α is some constant, and draw a graph of the dependence E from k(Fig. 12.1). We will call such a graph an “energy diagram.” An electron in a certain state of energy and momentum can be represented on such a graph by a point (S
in the figure).
We have already mentioned in Chap. 11 that the same state of affairs will arise if we we'll remove it electron from a neutral insulator. Then an electron from a neighboring atom can jump to this place. He will fill the “hole”, and he will leave a new “hole” in the place where he stood. We can describe this behavior by specifying the amplitude of what hole will be near this particular atom, and saying that hole can jump from atom to atom. (And it is clear that the amplitude A that the hole jumps over the atom A to the atom b, exactly equal to the amplitude of that electron from the atom b jumps into the hole from the atom A.)
Mathematics for holes is the same as for the additional electron, and we again find that the energy of the hole is related to its wave number by an equation that exactly coincides with (12.1) and (12.2), but, of course, with others numerical values amplitudes Ah x,A y And A z. A hole also has energy associated with the wavenumber of its probability amplitudes. Its energy lies in a certain limited zone and, near the bottom of the zone, changes quadratically with increasing wave number (or momentum) in the same way as in Fig. 12.1. Repeating our reasoning in Chap. 11, §3, we will find that the hole also behaves like a classical particle with a certain effective mass, the only difference being that in non-cubic crystals the mass depends on the direction of movement. So, the hole resembles put a particlebody charge, moving through the crystal. The charge of a hole particle is positive because it is concentrated in a place where there is no electron; and when it moves in some direction, it is actually in reverse side electrons are moving.
If several electrons are placed in a neutral crystal, their movement will be very similar to the movement of atoms in a gas under low pressure. If there are not too many of them, their interaction can be neglected. If you then apply an electric field to the crystal, the electrons will begin to move and an electric current will flow. In principle, they should end up at the edge of the crystal and, if there is a metal electrode there, move to it, leaving the crystal neutral.
In the same way, many holes could be introduced into the crystal. They would start wandering around randomly. If an electric field is applied, they will flow to the negative electrode and can then be “removed” from it, which is what happens when they are neutralized by electrons from the metal electrode.
Electrons and holes can appear in the crystal at the same time. If there are not too many of them again, then they will wander independently. In an electric field, they will all contribute to the total current. By obvious reason electrons are called negative carriers, and the holes - positive carriers.
 Until now, we believed that electrons were introduced into the crystal from the outside or (to form a hole) removed from it. But you can also “create” an electron-hole pair by removing a bound electron from a neutral atom and placing it in the same crystal at some distance. Then we get a free electron and free hole, and their movement will be as we described.
Until now, we believed that electrons were introduced into the crystal from the outside or (to form a hole) removed from it. But you can also “create” an electron-hole pair by removing a bound electron from a neutral atom and placing it in the same crystal at some distance. Then we get a free electron and free hole, and their movement will be as we described.
 The energy required to place an electron in the state S
(we say: to “create” a state S),
is energy E¯, shown in Fig. 12.2. This is some energy that exceeds E¯ min. The energy required to "create" a hole in some state S′,
is energy E+(Fig. 12.3), which is some fraction higher than E(=E + min).
The energy required to place an electron in the state S
(we say: to “create” a state S),
is energy E¯, shown in Fig. 12.2. This is some energy that exceeds E¯ min. The energy required to "create" a hole in some state S′,
is energy E+(Fig. 12.3), which is some fraction higher than E(=E + min).
 And to create a couple in states S
And S′,
you just need energy E¯+ E+.
And to create a couple in states S
And S′,
you just need energy E¯+ E+.
Pair formation is, as we will see later, a very common process, and many people choose to place figs. 12.2 and 12.3 per drawing, and energy holes postpone down, although, of course, this energy positive. In fig. In Figure 12.4 we combined these two graphs. The advantage of such a graph is that the energy E of the pair = E¯+ E+,
required to form a pair (electron in S
and holes in S′
),
is simply given by the vertical distance between S
And S′
,
as shown in Fig. 12.4. The smallest energy required to form a pair is called the energy width, or gap width, and is equal to
 Sometimes you may come across a simpler diagram. It is drawn by those who are not interested in the variable k, calling it a diagram energy levels. This diagram (shown in Fig. 12.5) simply indicates the permissible energies of electrons and holes.
Sometimes you may come across a simpler diagram. It is drawn by those who are not interested in the variable k, calling it a diagram energy levels. This diagram (shown in Fig. 12.5) simply indicates the permissible energies of electrons and holes.
How is an electron-hole pair created? There are several ways. For example, light photons(or x-rays) can be absorbed and form a pair if only the photon energy is greater than the energy width. The rate of pair formation is proportional to the light intensity. If you press two electrodes to the ends of the crystal and apply a “bias” voltage, then electrons and holes will be attracted to the electrodes. The current in the circuit will be proportional to the light intensity. This mechanism is responsible for the phenomenon of photoconductivity and for the operation of photocells. Electron-hole pairs can also be formed by particles high energies. When a fast moving charged particle (for example, a proton or pion with an energy of tens or hundreds Mev) flies through a crystal, its electric field can rip electrons from their bound states, forming electron-hole pairs. Hundreds and thousands of similar phenomena occur on every millimeter of the trace. After the particle passes, the carriers can be collected and thereby cause electrical impulse. Here is the mechanism of what is played out in semiconductor counters, in lately used in experiments on nuclear physics. For such counters, semiconductors are not needed; they can be made from crystalline insulators. This is what actually happened: the first of these counters was made of diamond, which is an insulator at room temperatures. But we need very pure crystals if we want electrons and holes to be able to reach the electrodes without fear of being captured. This is why silicon and germanium are used, because samples of these semiconductors of reasonable size (on the order of a centimeter) can be obtained of great purity.
So far we have only touched on the properties of semiconductor crystals at temperatures around absolute zero. At any non-zero temperature, there is another mechanism for creating electron-hole pairs. Can provide energy to a couple thermal energy crystal. Thermal vibrations of the crystal can transfer their energy to the pair, causing the “spontaneous” birth of pairs.
The probability (per unit time) that energy reaching the energy gap E gap will be concentrated at the location of one of the atoms is proportional to exp (—E gap /xT), Where T is the temperature, and x is Boltzmann’s constant [see Ch. 40 (issue 4)]. Near absolute zero, this probability is little noticeable, but as the temperature rises, the probability of the formation of such pairs increases. The formation of vapor at any finite temperature must continue without end, giving all the time with constant speed more and more positive and negative carriers. Of course, this won't actually happen, because after a moment the electrons will accidentally meet the holes again, the electron will roll into the hole, and the released energy will go to the lattice. We will say that the electron and hole are “annihilated.” There is a certain probability that a hole will meet an electron and both of them will destroy each other.
If the number of electrons per unit volume is Nn
(n means negative, or negative, carriers), and the density of positive (positive) carriers N p, then the probability that an electron and a hole will meet and annihilate per unit time is proportional to the product N n N p. At equilibrium, this rate must be equal to the rate at which pairs are formed. Therefore, at equilibrium the product NnNp
must be equal to the product of some constant and the Boltzmann factor
When we talk about constant, we mean its approximate constancy. More complete theory, which takes into account various details of how electrons and holes “find” each other, indicates that the “constant” also slightly depends on temperature; but the main dependence on temperature is still exponential.
Let's take for example pure substance, which was originally neutral. At a finite temperature one can expect that the number of positive and negative carriers will be the same, Nn = N r. This means that each of these numbers should change with temperature as e - E slots / 2xT. The change in many properties of a semiconductor (for example, its conductivity) is determined mainly by the exponential factor, because all other factors depend much less on temperature. The gap width for germanium is approximately 0.72 ev, and for silicon 1.1 ev.
At room temperature xT is about 1/4o ev. At these temperatures there are already enough holes and electrons to provide noticeable conductivity, whereas at, say, 30°K (one tenth of room temperature) conduction is undetectable. The slot width of a diamond is 6-7 ev, Therefore, at room temperature, diamond is a good insulator.
In many educational institutions And in offices, it’s not uncommon to come across such a convenient tool for work as a magnetic marker board 90 120. This is truly an indispensable assistant in conducting classes, trainings, and presentations. Such a board will allow you to clearly display a long formula in physics, or build a graph or diagram.
To describe electronic phenomena in the valence band that is not completely filled with electrons. IN electronic spectrum In the valence band, several zones often appear, differing in the effective mass and energy position (zones of light and heavy holes, zone of spin-orbit split holes).
Doping crystals with acceptor impurities is used to create holes in semiconductors. In addition, holes can also appear as a result of external influences: thermal excitation of electrons from the valence band to the conduction band, illumination with light.
In the case of Coulomb interaction of a hole with an electron from the conduction band, a bound state called an exciton is formed.
Wikimedia Foundation. 2010.
See what “Hole (charge carrier)” is in other dictionaries:
Charge carriers common name mobile particles or quasiparticles that carry electric charge and are capable of ensuring the flow electric current. Examples of mobile particles are electrons and ions. An example of a charge carrier quasiparticle... ... Wikipedia
In physics, a quantum state not occupied by an electron. The term hole is widely used in band theory solid body as a vacant state in the allowed filled zone. A hole is a positively charged charge carrier in a semiconductor... Big Encyclopedic Dictionary
AND; pl. genus. rock, date rkam; and. 1. = Hole (1 2 digits). Holes in the walls. In the back tooth d. Mend the hole. There is a huge number on the stocking. 2. A through hole for attaching something. Holes in the belt. D. for a screw. Drill, poke a hole. 3. Unlock About the bullet... Encyclopedic Dictionary
This term has other meanings, see Hole (meanings). It is necessary to check the quality of the translation and bring the article into compliance with the stylistic rules of Wikipedia. You can help... Wikipedia
GOST 22622-77: Semiconductor materials. Terms and definitions of basic electrophysical parameters- Terminology GOST 22622 77: Semiconductor materials. Terms and definitions of basic electrophysical parameters original document: 11. Acceptor A lattice defect capable of capturing an electron from the valence band when excited. Definitions... ... Dictionary-reference book of terms of normative and technical documentation
In va, characterized by an increase in electrical power. conductivity with increasing temperature. Although P. is often defined as in va with ud. electric conductivity a, intermediate between its values for metals (s! 106 104 Ohm 1 cm 1) and for good dielectrics (s! 10 ... Chemical encyclopedia
Observed at high concentrations of impurities. Their interaction leads to qualitative changes properties of semiconductors. This can be observed in heavily doped conductors containing impurities in such high concentrations Npr that the average ... ... Wikipedia
A wide class of substances characterized by electrical conductivity values σ intermediate between the electrical conductivity of metals (See Metals) (σ Semiconductors 106 104 ohm 1 cm 1) and good dielectrics (See Dielectrics) (σ ≤ 10 10 10 12 ohm... ... Great Soviet Encyclopedia
A wide class in, characterized by the values of beats. electrical conductivity s, intermediate between specifications. electrical conductivity of metals s = 106 104 Ohm 1 cm 1 and good dielectrics s = 10 10 10 12 Ohm 1 cm 1 (electrical conductivity is indicated at room temperature).… … Physical encyclopedia
Ov; pl. (unit semiconductor, a; m.). Phys. Substances that, in terms of electrical conductivity, occupy an intermediate position between conductors and insulators. Properties of semiconductors. Semiconductor production. // Electrical appliances and devices... ... Encyclopedic Dictionary
Since in solid body atoms or ions are brought together at a distance comparable to the size of the atom itself, then transitions of valence electrons from one atom to another occur in it. This electronic exchange can result in the formation of a covalent bond. This occurs when the electron shells of neighboring atoms overlap greatly and electron transitions between atoms occur quite often.
This picture is entirely applicable to a typical semiconductor such as germanium (Ge). All germanium atoms are neutral and bonded to each other by covalent bonds. However, electron exchange between atoms does not directly lead to electrical conductivity, since in general the distribution of electron density is rigidly fixed: 2 electrons per bond between each pair of atoms - nearest neighbors. To create conductivity in such a crystal, it is necessary to break at least one of the bonds (heating, photon absorption, etc.), that is, by removing an electron from it, transfer it to some other cell of the crystal, where all the bonds are filled and this the electron will be redundant. Such an electron can subsequently freely move from cell to cell, since they are all equivalent for it, and, being superfluous everywhere, it carries with it the excess negative charge, that is, it becomes a conduction electron.
A broken bond becomes a hole wandering around the crystal, since under conditions of strong exchange an electron from one of the neighboring connections quickly takes the place of the one who left, leaving the connection from which he left broken. The lack of an electron on one of the bonds means that the atom (or pair of atoms) has a single positive charge, which is thus transferred along with the hole.
In the case of ionic bonding, overlap electronic shells less, electronic transitions less frequent. When a bond is broken, a conduction electron and a hole are also formed - an extra electron in one of the crystal cells and an uncompensated positive charge in another cell. Both of them can move around the crystal, moving from one cell to another.
The presence of two oppositely charged types of current carriers - electrons and holes - is common property semiconductors and dielectrics. In ideal crystals, these carriers always appear in pairs - the excitation of one of the bound electrons and its transformation into a conduction electron inevitably causes the appearance of a hole, so that the concentrations of both types of carriers are equal. This does not mean that their contribution to electrical conductivity is the same, since the rate of transition from cell to cell (mobility) for electrons and holes can be different. IN real crystals containing impurities and structural defects, the equality of the concentrations of electrons and holes may be violated, so that electrical conductivity in this case will be carried out practically only by one type of carriers.
In the section on the question What is electron hole? given by the author Virus. the best answer is But it seems to me that this is something that “moves” in the opposite direction from the movement of electrons, and is positively charged. This is a generalization. Used in semiconductors.
Read here:
Source: The absence of an electron in a semiconductor atom is conventionally called a hole. It should be borne in mind that a hole is not a particle, but a place vacated after an electron. The hole behaves like an elementary positive (namely positive) charge.
Reply from Helga[guru]
If the semiconductor is pure (without impurities), then it has its own conductivity, which is low. There are two types of intrinsic conductivity:
1) electronic (conductivity "n" - type)
At low temperatures in semiconductors, all electrons are bound to the nuclei and the resistance is high; with increasing temperature kinetic energy particles increases, bonds break down and free electrons appear - the resistance decreases.
Free electrons move opposite to the electric voltage vector. fields.
Electronic conductivity of semiconductors is due to the presence of free electrons.
2) hole (conductivity "p" - type)
When the temperature increases, they break down covalent bonds carried out valence electrons, between the atoms, spaces with a missing electron are formed - a “hole”.
It can move throughout the crystal, because its place can be replaced by valence electrons. Moving a "hole" is equivalent to moving a positive charge.
The hole moves in the direction of the electric field strength vector.
Reply from &Ъ[guru]
An atom that is missing an electron, to put it simply.
Reply from Sc@r[newbie]
there is no such thing!
Reply from S.Forget[guru]
This place is in crystal lattice, where there is a missing electron. It is conventionally accepted to consider a hole to be positive, although in reality there is no movement of holes - it is the electrons that move, filling the holes. At the same time, where the electron “escaped” from, a hole remains. This creates the appearance of “movement” of positive carriers - holes, that is.
In short, the voids in the lattice are holes, and they attract electrons. Therefore, holes are considered positive