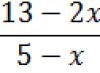Electric current in metals
Metals are good conductors of electricity. This is due to their internal structure. All metals have outer valence electrons weakly bound to the nucleus, and when atoms combine into a crystal lattice, these electrons become common, belonging to the entire piece of metal.

Charge carriers in metals are electrons .
Electrons in metals, when placed in an electric field, move with a constant average speed proportional to the field strength.

Dependence of conductor resistance on temperature
As the temperature increases, the speed of thermal movement of conduction electrons increases, which leads to an increase in the frequency of collisions with ions of the crystal lattice and, thereby, to an increase in resistance.
Superconductivity - the phenomenon of a sharp decrease in conductor resistance to zero when cooled to critical temperature(depending on the type of substance).
Superconductivity is a quantum effect. It is explained by the fact that when low temperatures macroscopic number of electrons behave as a single object. They cannot exchange portions of energy with the crystal lattice that are less than their binding energy, so thermal energy dissipation does not occur, which means the absence of resistance.
Such a combination of electrons is possible when they form bosonic (Cooper) pairs - a correlated state of electrons with opposite spins and momenta.


The Meissner effect is the displacement of a magnetic field from a superconductor. Undamped currents circulate inside the conductor in a superconducting state, creating a magnetic field opposite to the external one. A strong magnetic field destroys superconductivity.

Electric current in liquids
Electrolytes it is customary to call conducting media in which the flow of electric current is accompanied by the transfer of matter
Having reached the cathode, copper ions are neutralized by excess electrons of the cathode and turn into neutral atoms that are deposited on the cathode. Chlorine ions, reaching the anode, give up one electron each. Chlorine is released at the anode in the form of bubbles.
The law of electrolysis was experimentally established by the English physicist M. Faraday in 1833 ( Faraday's law)

m- mass of pure substance released as a result of electrolysis
k- electric chemical equivalent substances
Here N A- Avogadro's constant, M = m 0 N A- molar mass of substance,
F = eN A =96485 C/mol- Faraday's constant
Faraday's constant is numerically equal to the charge that must be passed through the electrolyte to release one mole of a monovalent substance at the electrode
Faraday's law for electrolysis


Electric current in gases
Under normal conditions, all gases are dielectrics, that is, they do not conduct electric current. This property explains, for example, the widespread use of air as an insulating substance. The principle of operation of switches and circuit breakers is precisely based on the fact that by opening their metal contacts, we create a layer of air between them that does not conduct current.
However, under certain conditions, gases can become conductors. For example, a flame introduced into the space between two metal disks (see figure) causes the galvanometer to note the appearance of a current. The conclusion follows: a flame, that is, a gas heated to a high temperature, is a conductor of electric current.

Heating - no the only way transforming gas into a conductor. Instead of flame, you can use ultraviolet or x-ray radiation, as well as the flow of alpha particles or electrons. Experiments have established that the action of any of these causes leads to the ionization of gas molecules.
The passage of current through gases is called a gas discharge. We have just looked at an example of a so-called non-self-sustaining discharge. It is so called because it requires some kind of ionizer to maintain it - flame, radiation or a stream of charged particles. Experiments show that if the ionizer is removed, the ions and electrons soon reunite (they say: recombine), again forming electrically neutral molecules. As a result, the gas stops conducting current, that is, it becomes a dielectric.
Independent and non-independent conductivity of gases
In order to make a gas conductive, it is necessary in one way or another to introduce into it or create in it free charge carriers - charged particles. In this case, two cases are possible: either these charged particles are created by the action of some external factor or are introduced into the gas from the outside - non-self-conductivity, or they are created in the gas by the action of the electric field, existing between the electrodes - independent conductivity.
In the case of non-self-sustained conductivity, at small values of U, the graph looks like a straight line, i.e. Ohm's law approximately remains in force; As U increases, the curve bends with some tension and turns into a horizontal straight line.
This means that starting from a certain voltage, the current remains constant despite the voltage increasing. This constant, voltage-independent current value is called saturation current.
Non-self-sustaining gas discharge - a discharge that exists only under the influence of external ionizers.
As the voltage increases, impact ionization occurs - the phenomenon of knocking out electrons from neutral molecules - the number of charge carriers increases like an avalanche. An independent discharge occurs.
Self-sustaining gas discharge - discharge that exists after removal of external ionizers.
Processes affecting the conductivity of gases
|
Thermal ionization- when neutral atoms collide, electrons are knocked out and atoms transform into positive ions |

|
|
Ionization by radiation(photoionization) - the decay of an atom into an electron and a positive ion under the influence of light |

|
|
Electron impact ionization- knocking out an electron from an atom by an accelerated electron to form a positive ion |

|
|
Secondary electron emission from the cathode - knocking out electrons from the cathode by positive ions |

|
|
Thermionic emission- emission of electrons by heated metal |

|
Glow discharge: At a gas pressure of several tenths of a millimeter of mercury, the discharge has a typical form, schematically shown in Fig. This is the current in ionized gas, or more precisely in low-temperature plasma. A glow discharge is formed when current passes through a discharged gas. As soon as the voltage exceeds a certain value, the gas in the flask ionizes and glow occurs. This is essentially an electric current not so much in a gas as in a plasma. The color of the gas (plasma) glow depends on the substance of the gas.
Spark discharge: At a sufficiently high field strength (about 3 MV/m), an electric spark appears between the electrodes, which has the appearance of a brightly glowing winding channel connecting both electrodes. The gas near the spark heats up to a high temperature and suddenly expands, causing sound waves, and we hear a characteristic crack. Occurs under normal conditions, under normal conditions atmospheric pressure, just like a glow discharge occurs as a result of gas ionization, but at high voltage, unlike an arc discharge, where it is primarily important high density current
Corona discharge: occurs in a strong electric field with high intensity, sufficient to cause ionization of the gas (or liquid). In this case, the electric field is not uniform; in some places the intensity is much greater. A gradient (difference) of field potentials is formed, and where the potential is greater, the ionization of the gas occurs stronger, more intense, then the flow of ions reaches another part of the field, thereby forming a flow of electricity. As a result, a corona gas discharge of bizarre shapes is formed, depending on the geometry of the conductors - the sources of field strength.
Arc discharge: represents electrical breakdown gas, which later becomes a permanent plasma discharge - an arc, is formed electric arc. An arc discharge is characterized by a lower voltage than a glow discharge. Maintained mainly due to thermionic emission, when electrons are released from the electrodes. The old name for such an arc is “voltaic arc”. Distinctive feature Such an arc is characterized by high current density and low voltage, which is limited by the current source. In order to create such an arc, the electrodes are brought closer together, a breakdown occurs, and then they move apart.
Experience shows that two differently charged plates separated by a layer of air do not discharge.
Typically, a substance in the gaseous state is an insulator because the atoms or molecules of which it is composed contain same number negative
and positive electric charges and are generally neutral.
Let's bring the flame of a match or spirit lamp into the space between the plates (Fig. 164). In this case, the electrometer will begin to discharge quickly. Consequently, the air under the influence of the flame became a conductor. When the flame is removed from the space between the plates, the discharge of the electrometer stops. The same result can be obtained by irradiating the plates with electric arc light. These experiments prove that gas can become a conductor of electric current.
The phenomenon of the passage of electric current through a gas, observed only under the condition of some external influence, is called a non-self-sustaining electrical discharge.
Thermal ionization.
Heating a gas makes it a conductor of electric current because some of the gas's atoms or molecules turn into charged ions.
To remove an electron from an atom, work must be done against the forces Coulomb attraction between a positively charged nucleus and a negative electron. The process of removing an electron from an atom is called ionization of the atom. The minimum energy that must be expended to remove an electron from an atom or molecule is called binding energy.
An electron can be torn from an atom when two atoms collide if their kinetic energy exceeds the binding energy of the electron. The kinetic energy of thermal motion of atoms or molecules is directly proportional absolute temperature Therefore, with increasing gas temperature, the number of collisions of atoms or molecules, accompanied by ionization, increases.
Emergence process free electrons and positive ions resulting from collisions of atoms and gas molecules at high temperatures is called thermal ionization.
A gas in which a significant portion of the atoms or molecules are ionized is called plasma.
The degree of thermal ionization of plasma depends on temperature. For example, at a temperature of 10,000 K, less than 10% of the total number of hydrogen atoms is ionized; at temperatures above 20,000 K, hydrogen is almost completely ionized.
Plasma electrons and ions can move under the influence of an electric field. Thus, at low temperatures the gas is an insulator, at high temperatures turns into plasma and becomes a conductor of electric current.
Photoionization.
The energy required to remove an electron from an atom or molecule can be transferred by light. Ionization
atoms or molecules under the influence of light is called photoionization.
Self-contained electrical discharge.
When the electric field strength increases to a certain value, depending on the nature of the gas and its pressure, an electric current arises in the gas even without the influence of external ionizers. The phenomenon of electric current passing through a gas, independent of the action of external ionizers, is called an independent electric discharge.
In air at atmospheric pressure, an independent electric discharge occurs at an electric field strength equal to approximately
![]()
The main mechanism of gas ionization during an independent electric discharge is the ionization of atoms and molecules due to electron impacts.
Electron impact ionization.
Ionization by electron impact becomes possible when an electron, during its free path, acquires a kinetic energy that exceeds the binding energy of the electron with the atom.
The kinetic energy of an electron acquired under the influence of an electric field of intensity E is equal to the work done by the electric field forces:
![]()
where is the free path length.
Hence the approximate condition for the onset of ionization by electron impact has the form
The binding energy of electrons in atoms and molecules is usually expressed in electron volts (eV). 1 eV equal to work, which the electric field makes when moving an electron (or other particle with elementary charge) between field points, the voltage between which is 1 V:

The ionization energy of a hydrogen atom, for example, is 13.6 eV.
Self-discharge mechanism.
The development of an independent electric discharge in a gas proceeds as follows. A free electron under the influence of an electric field acquires acceleration. If the electric field strength is sufficiently high, the free path electron increases its kinetic energy so much that it ionizes it upon collision with a molecule.
The first electron, which caused the ionization of the molecule, and the second electron, released as a result of ionization, under the influence of an electric field acquire acceleration in the direction from the cathode to the anode. Each of them, during subsequent collisions, releases one more electron and total number free electrons becomes

equal to four. Then, in the same way, it increases to 8, 16, 32, 64, etc. The number of free electrons moving from the cathode to the anode increases like an avalanche until they reach the anode (Fig. 165).
Positive ions formed in the gas move under the influence of an electric field from the anode to the cathode. When positive ions strike the cathode and under the influence of light emitted during the discharge process, new electrons can be released from the cathode. These electrons, in turn, are accelerated by the electric field and create new electron-ion avalanches, so the process can continue continuously.
The concentration of ions in the plasma increases as the self-sustained discharge develops, and the electrical resistance of the discharge gap decreases. The current strength in a self-discharge circuit is usually determined only by the internal resistance of the current source and the electrical resistance of other elements of the circuit.
Spark discharge. Lightning.
If the current source is not capable of maintaining a self-sustained electrical discharge for a long time, then the self-sustained discharge that occurs is called a spark discharge. The spark discharge stops a short period of time after the start of the discharge as a result of a significant decrease in voltage. Examples of spark discharge are sparks that occur when combing hair, separating sheets of paper, or discharging a capacitor.
Lightning observed during a thunderstorm also represents an independent electrical discharge. The current strength in the lightning channel reaches , the duration of the current pulse is several tens of microseconds. An independent electrical discharge between a thundercloud and the Earth stops by itself after several lightning strikes, since most of the excess electrical charges in the thundercloud are neutralized by the electric current flowing through the lightning plasma channel (Fig. 166).
When the current in the lightning channel increases, the plasma heats up to a temperature above. Changes in pressure in the lightning plasma channel when the current increases and the discharge stops causes sound phenomena called thunder.

Glow discharge.
As the gas pressure in the discharge gap decreases, the discharge channel becomes wider, and then the entire discharge tube is uniformly filled with luminous plasma. This type of independent electrical discharge in gases is called a glow discharge (Fig. 167).
Electric arc.
If the current strength in a self-sustained gas discharge is very high, then impacts from positive ions and electrons can cause heating of the cathode and anode. At high temperatures, electrons are emitted from the surface of the cathode, ensuring the maintenance of an independent discharge in the gas. A long-term independent electrical discharge in gases, maintained by thermionic emission from the cathode, is called an arc discharge (Fig. 168).


Corona discharge.
In highly inhomogeneous electric fields formed, for example, between a tip and a plane or between a wire and a plane (power line), an independent discharge of a special type occurs, called a corona discharge. During a corona discharge, ionization by electron impact occurs only near one of the electrodes, in an area with high electric field strength.
Application of electrical discharges.
Impacts of electrons accelerated by an electric field lead not only to the ionization of gas atoms and molecules, but also to
excitation of atoms and molecules, accompanied by the emission of light. Light emission from plasma of self-sustained electric discharge is widely used in national economy and in everyday life. These are fluorescent lamps and gas-discharge lamps for street lighting, an electric arc in a film projection apparatus and mercury-quartz lamps used in hospitals and clinics.
The high temperature of the arc discharge plasma allows it to be used for cutting and welding metal structures and for melting metals. Using a spark discharge, parts made from the hardest materials are processed.
Electrical discharge in gases can also be an undesirable phenomenon that must be combated in technology. For example, a corona electrical discharge from the wires of high-voltage power lines leads to useless losses of electricity. The increase in these losses with increasing voltage puts a limit on the path to further increasing the voltage in the power line, whereas such an increase is highly desirable to reduce energy losses due to heating of wires.
Let us assume that the gas under study is enclosed in a vessel C with two electrodes, to which a potential difference is applied. The electric field between the electrodes can be changed by moving the potentiometer slide that closes the battery (Fig. III.42). If there are no free charges in the gas (positive or negative ions or electrons), then there will be no current in the galvanometer circuit. Note that gases will always contain a certain amount of charges, since gas is ionized both during inevitable thermal collisions of molecules and under the influence of various radiations, in particular,
from radioactive substances. However, simultaneously with the process of ionization, i.e., the separation of neutral molecules into charged ions, the reverse process of molization or recombination occurs in the gas, i.e., the combination of ions into neutral molecules. IN equilibrium state both of these gases. processes are balanced: the number of molecules ionizing every second is equal to the number of neutral molecules newly formed from ions during the same time.
If there is no external ionizing effect on the gas, then the natural concentration of ions in it will be very small, and the current through the gas will be practically undetectable. It is possible to cause a noticeable electric current in a gas (the so-called gas discharge) if: 1) with the help of an external influence (an ionizer), you continuously break neutral gas molecules into ions and thereby increase the concentration of free charges in the gas. This can be done by exposing the gas to intense irradiation with a flow of fast particles (electrons, etc.), ultraviolet, x-rays, rays of radioactive substances, as well as increasing the temperature of the gas to increase the intensity of ionization during thermal collisions. In this case, along with the termination of the external ionizer, the current through the gases also stops; such conductivity of a gas is called non-self-sustaining; 2) apply such a large potential difference that the ions present in the gas, accelerating in the electric field, acquire energies sufficient to ionize neutral molecules upon collisions with them. In this case, each ion in one collision causes the appearance of two or more ions; these ions, in turn, are accelerated in the field and break neutral molecules into ions. Thus, the number of ions in the gas increases rapidly, and the gas acquires noticeable conductivity; such conductivity is called independent.

It is necessary to distinguish between two types of collisions between particles, in particular between ions, electrons and neutral molecules. In some collisions the particles do not experience any internal changes, but only exchange kinetic energies of movement. Such collisions are called elastic; the sum of the kinetic energies of the particles before and after the impact remains constant.
In other - inelastic - collisions, atoms and molecules experience changes in their structure; there is a transition of the kinetic energy of colliding particles into the potential energy of interaction between the constituent parts of these atoms and molecules - nuclei and electrons rotating around them. This process is called excitation of atoms or molecules; when going back to normal condition the absorbed energy is returned as radiant energy. Finally, when inelastic collisions it's also possible
changes in the composition of atoms and molecules; in particular, a neutral molecule can be broken into two ions or an electron can be torn away from an atom, etc. Ionization of gases during collisions is the result of inelastic collisions.
For the conductivity of gases, under certain conditions (in particular, at low gas pressures in the vessel), the knocking out of electrons from the surface of the cathode when positive ions fall on it is of significant importance. Each such ion can release several electrons from the cathode, depending on the energy acquired by it in the electric field, as well as on the work function of the electron from the cathode substance. Electrons released from the cathode, picked up by the electric field, can cause ionization of the gas on their way to the anode; in addition, this ordered flow of electrons constitutes a certain (sometimes significant) fraction of the total current flowing through the gas:
If the current passing through the gases is small and cannot be directly detected by a galvanometer, then resort to indirect methods. In particular, as shown in Fig. III.42, a resistor with a resistance of the order of tens and hundreds of millions of ohms is connected to the circuit in series with the gas gap. A potential difference is formed at the ends of this resistor, which is measured, for example, with a lamp voltmeter that does not short the ends of this resistor. Then, knowing and measuring, you can calculate the current strength through the gas. For example, if , then
Electric current in gases and liquids
Electric current in gases
Charge carriers: electrons, positive ions, negative ions.
Charge carriers appear in the gas as a result of ionization: due to irradiation of the gas, or collisions of heated gas particles with each other.
Electron impact ionization.

E – field direction;
l is the mean free path between two successive collisions of an electron with gas atoms.
A_=eEl\geq W – ionization condition
W – ionization energy, i.e. energy required to remove an electron from an atom

The number of electrons increases exponentially, resulting in an electron avalanche and, consequently, a discharge in the gas.
Electric current in liquid
Liquids as well as solids can be dielectrics, conductors and semiconductors. Dielectrics include distilled water, conductors include solutions of electrolytes: acids, alkalis, salts and molten metals. Liquid semiconductors are molten selenium and sulfide melts.
When electrolytes dissolve under the influence of the electric field of polar water molecules, the electrolyte molecules disintegrate into ions. For example, CuSO_ \rightarrow Cu^ +SO^ _ .
Along with dissociation, the reverse process occurs - recombination, i.e. combining ions of opposite signs into neutral molecules.
The carriers of electricity in electrolyte solutions are ions. This conductivity is called ionic .
If electrodes are placed in a bath with an electrolyte solution and current is applied, then negative ions will move to the positive electrode, and positive ions to the negative.
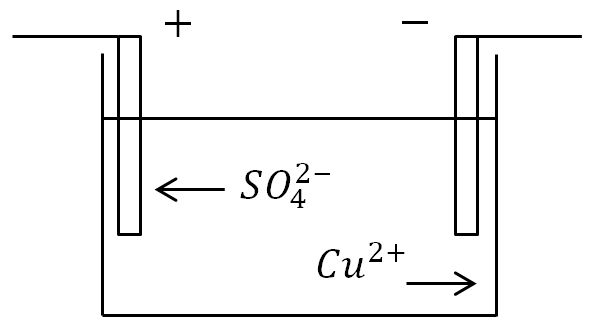
At the anode (positive electrode), negatively charged ions give up extra electrons (oxidation reaction), and at the cathode (negative electrode), positive ions receive the missing electrons (reduction reaction).
Definition. The process of releasing substances on electrodes associated with redox reactions is called electrolysis.
Faraday's laws
I. The mass of the substance that is released on the electrode is directly proportional to the charge flowing through the electrolyte:
k is the electrochemical equivalent of the substance.
q=I\Delta t , then
\frac – chemical equivalent of the substance;
\mu – molar mass;
Electrochemical equivalents of substances are proportional to chemical ones.
F – Faraday constant;
Unified law of electrolysis
Substituting k into the expression for m (Faraday’s First Law), we get:
Physical meaning of the electrochemical equivalent.

Electrochemical equivalent equal to the ratio the mass of the ion to its charge:

I started studying with Natalya Lvovna in mid-January, immediately after New Year holidays. Before the start of classes, there were trial exams in physics, as well as preparation for the exam at school, but the result was 60-70 points, while I received excellent grades in the subject. Classes with Natalya Lvovna were fruitful and interesting; with this tutor I was able to expand my knowledge of physics, as well as consolidate the school curriculum. Having completed the spring intensive courses, I entered the exam confident in my results. Having received 85 points, I was able to enter the desired university with 1 wave. I would like to once again thank the tutor who helped me get closer to my goals and pass the single state exam with the required scores, enroll in a university and begin training for your future profession.

Natalya Lvovna is a wonderful physics tutor who will perfectly prepare you for the Unified State Exam.
I didn’t come to her with zero knowledge, but I couldn’t call it good. Although I started studying in January, we managed to cover all the topics in additional classes.
Each topic was analyzed and all types of problems that could be encountered in the exam were solved.
And indeed, at the Unified State Exam I did not encounter any difficulties in solving problems and wrote the exam with 94 points.
I highly recommend this teacher!

My daughter Polina studied at a school with a “humanitarian bias”. The main subjects for her from the first grade were foreign languages. But when the question of choosing a profession arose, the daughter wanted to enter technical university. It is obvious that the school curriculum is not rubber, and it is not surprising that at 8 school hours foreign languages she only had one physics lesson a week. I had to urgently look for a solution. We were lucky - we found a wonderful tutor.
Natalya Lvovna was fully able to prepare Polina for the exam. For our humanitarian school, 85 points in physics is an excellent result. We are very grateful - Natalya Lvovna is an excellent teacher and a sensitive person. Individual approach to everyone even group classes– this is what I would like to note first. We hope to enroll in your dream university.
ELECTRIC CURRENT IN GASES
Under normal conditions, gas is a dielectric, i.e. it consists of neutral atoms and molecules and does not contain free carriers of electric current.
The conductor gas is an ionized gas. Ionized gas has electron-ion conductivity.
Air is a dielectric in power lines, air capacitors, and contact switches.
Air is a conductor when lightning occurs, electric spark, when a welding arc occurs.

is the disintegration of neutral atoms or molecules into positive ions and electrons by removing electrons from the atoms. Ionization occurs when a gas is heated or exposed to radiation (UV, X-rays, radioactive) and is explained by the disintegration of atoms and molecules during collisions at high speeds.
- this is electric current in ionized gases.
The charge carriers are positive ions and electrons. Gas discharge is observed in gas-discharge tubes (lamps) when exposed to an electric or magnetic field.


Recombination of charged particles

- the gas ceases to be a conductor if ionization stops, this occurs as a result of recombination (reunion of oppositely charged particles).
There is a self-sustaining and non-self-sustaining gas discharge.
Non-self-sustaining gas discharge
- if the action of the ionizer is stopped, the discharge will also stop.
When the discharge reaches saturation, the graph becomes horizontal. Here, the electrical conductivity of the gas is caused only by the action of the ionizer.
Self-sustaining gas discharge
— in this case, the gas discharge continues even after the termination of the external ionizer due to ions and electrons resulting from impact ionization (= ionization of electric shock); occurs when the potential difference between the electrodes increases (an electron avalanche occurs).
A non-self-sustained gas discharge can transform into a self-sustained gas discharge when Ua = Uignition.
Electrical breakdown of gas
— the process of transition of a non-self-sustaining gas discharge into a self-sustaining one.
Self-sustained gas discharge occurs 4 types:
1. smoldering - at low pressures (up to several mm Hg) - observed in gas-light tubes and gas lasers.
2. spark - at normal pressure and high electric field strength (lightning - current strength up to hundreds of thousands of amperes).
3. corona - at normal pressure in a non-uniform electric field (at the tip).
4. arc - high current density, low voltage between the electrodes (gas temperature in the arc channel -5000-6000 degrees Celsius); observed in spotlights and projection film equipment.
These discharges are observed:
smoldering - in fluorescent lamps;
spark - in lightning;
corona - in electric precipitators, during energy leakage;
arc - during welding, in mercury lamps.
- this is the fourth state of aggregation of matter with high degree ionization due to the collision of molecules on high speed at high temperature; found in nature: the ionosphere is a weakly ionized plasma, the Sun is a fully ionized plasma; artificial plasma - in gas-discharge lamps.
Low temperature - at temperatures less than 100,000K;
high temperature - at temperatures above 100,000K.
Basic properties of plasma:
– high electrical conductivity
— strong interaction with external electric and magnetic fields.
At temperature ![]()
Any substance is in a plasma state.
Interestingly, 99% of the matter in the Universe is plasma.
Other pages on the topic “Electricity” for grades 10-11:
class-fizika.narod.ru
Laws of electric current in gases
Official website of ANO DO Center "Logos", Glazov
GET READY FOR THE LESSON
Electric current in various environments, a little about physics:
Electric current is any ordered movement of electric charges. Electric current can pass through various substances under certain conditions. One of the conditions for the occurrence of electric current is the presence of free charges that can move under the influence of an electric field.
Therefore, in this section we will try to establish which particles carry electric charge in different media.
Electric current in metals.
Metals consist of positively charged ions located at the sites of a crystal lattice and a collection of free electrons. Outside an electric field, free electrons move chaotically, like molecules ideal gas, and therefore are considered in the classical electron theory How electron gas .
Under the influence of an external electric field, the nature of the movement of free electrons inside the metal changes. Electrons, continuing their chaotic movements, at the same time shift in the direction of the electric field forces.
Hence, electric current in metals is the ordered movement of electrons.
Current strength in a metal conductor determined by the formula:
![]()
Where I- current strength in the conductor, e— electron charge modulus, n 0 — concentration of conduction electrons, — average speed of ordered movement of electrons, S
The conduction current density is numerically equal to the charge passing through a unit surface area perpendicular to the direction of the current in 1 s.
![]()
Where j— current density.
In most metals, almost every atom is ionized. And since the concentration of conduction electrons of a monovalent metal is equal to

Where N a- Avogadro's constant, A— atomic mass metal, ρ - metal density,
then we find that the concentration is determined within the range of 10 28 - 10 29 m -3.
Ohm's law for a homogeneous section of a chain:
Where U- voltage in the area, R— resistance of the area.
For a homogeneous chain section:
Where ρ U- specific resistance of the conductor, l — conductor length, S- cross-sectional area of the conductor.
The resistivity of a conductor depends on temperature and this dependence is expressed by the relation:
Where ρ ou - resistivity of a metal conductor at temperature T = 273K, α — thermal coefficient of resistance, ∆T = T - T o - temperature change.

Current-voltage characteristics of metals.
According to Ohm's law, the current strength in conductors is directly proportional to the voltage. This dependence occurs for conductors with a strictly specified resistance ( for resistors).

The tangent of the slope of the graph is equal to the conductivity of the conductor. Conductivity called the reciprocal of resistance
But since the resistance of metals depends on temperature, the current-voltage characteristic of metals is not linear.

Electric current in solutions and melts of electrolytes.
The phenomenon of decomposition of molecules of salts, alkalis and acids in water into ions of opposite signs is called electrolytic dissociation. The ions resulting from the decay serve as charge carriers in the liquid, and the liquid itself becomes a conductor.

Outside the electric field, the ions move chaotically. Under the influence of an external electric field, the ions, continuing their chaotic movements, are at the same time displaced in the direction of the electric field forces: cations to the cathode, anions to the anode.
Hence, electric current in solutions (melts) of electrolytes is the directed movement of ions of both signs in opposite directions.
The passage of an electric current through an electrolyte solution is always accompanied by the release of substances included in its composition on the electrodes. This phenomenon is called electrolysis .
When moving inside electrolytes, ions interact with water molecules and other ions, i.e. electrolytes exert some resistance to movement and, therefore, have resistance. The electrical resistance of electrolytes depends on the concentration of ions, the magnitude of the charge of the ion, and the speed of movement of ions of both signs.
The resistance of electrolytes is also determined by the formula:
Where ρ U— specific resistance of the electrolyte, l — liquid conductor length, S is the cross-sectional area of the liquid conductor.
As the temperature of the electrolyte increases, its viscosity decreases, which leads to an increase in the speed of ion movement. Those. As the temperature increases, the electrolyte resistance decreases.
1. The mass of the substance released at the electrode is directly proportional to the electrical charge passing through the electrolyte.
Where m- mass of substance released at the electrode, k- electrochemical equivalent, q- charge passing through the electrolyte.
2. The electrochemical equivalent of a substance is directly proportional to its chemical equivalent.

Where M- molar mass of the substance, F- Faraday's constant z- valency of the ion.
Faraday's constant is numerically equal to the charge that must pass through the electrolyte in order to release from it a mass of substance numerically equal to the chemical equivalent.
Faraday's combined law.


Electric current in gases.
At normal conditions gases consist of neutral molecules and are therefore dielectrics. Since the presence of charged particles is necessary to produce an electric current, the gas molecules must be ionized (electrons removed from the molecules). To ionize molecules it is necessary to expend energy - ionization energy, the amount of which depends on the type of substance. Thus, the ionization energy is minimal for alkali metal atoms, and maximum for inert gases.
Molecules can be ionized by heating a gas or irradiating it with various types of rays. Thanks to the additional energy, the speed of movement of molecules increases, the intensity of their thermal motion increases, and upon collision, individual molecules lose electrons, turning into positively charged ions.

Electrons that break away from a molecule can join neutral molecules, forming negatively charged ions.

Therefore, during ionization, three types of charge carriers appear: positive ions, negative ions and electrons.
Under the influence of an external electric field, ions of both signs and electrons move in the direction of the electric field forces: positive ions to the cathode, negative ions and electrons to the anode. Those. electric current in gases is the ordered movement of ions and electrons under the influence of an electric field.
Current-voltage characteristics of gases.
The dependence of current on voltage is expressed by the OABC curve.
In the section of the OA graph, the current strength obeys Ohm's law. At low voltage, the current strength is small, because ions moving at low speeds recombine without reaching the electrodes. As the voltage between the electrodes increases, the speed of directional movement of electrons and ions increases, so most of the charged particles reach the electrodes, and, consequently, the current increases.

At a certain voltage value U1, all ions have sufficient velocities and, without recombining, reach the electrodes. The current becomes the maximum possible and does not depend on a further increase in voltage to the value U 2. This current is called saturation current, and the graph section AB corresponds to it.
At a voltage U 2 of several thousand volts, the speed of electrons arising from the ionization of molecules, and therefore their kinetic energy, increases significantly. And when the kinetic energy reaches the ionization energy value, electrons colliding with neutral molecules ionize them. Additional ionization leads to an avalanche-like increase in the number of charged particles, and consequently to a significant increase in current strength without the influence of an external ionizer. The passage of electric current without the influence of an external ionizer is called independent discharge. This dependence is expressed by the section of the AC graph.
Electric current in a vacuum.
There are no charged particles in a vacuum, and therefore it is a dielectric. Those. it is necessary to create certain conditions that will help produce charged particles.
There are free electrons in metals. At room temperature, they cannot leave the metal, because they are held in it by the forces of Coulomb attraction from positive ions. To overcome these forces, the electron must expend a certain energy, which is called work function. Energy, great or equal to work release, electrons can be obtained when the metal is heated to high temperatures.

When a metal is heated, the number of electrons with kinetic energy, more work exit, increases, so it flies out of the metal more electrons. The emission of electrons from metals when heated is called thermionic emission. To carry out thermionic emission, a thin wire filament made of refractory metal (incandescent filament) is used as one of the electrodes. A filament connected to a current source becomes hot and electrons fly out from its surface. The emitted electrons enter the electric field between the two electrodes and begin to move directionally, creating an electric current.
The phenomenon of thermionic emission underlies the operating principle of electron tubes: vacuum diode, vacuum triode.
Vacuum diode Vacuum triode


Current-voltage characteristic of a vacuum diode.
The dependence of current on voltage is expressed by the OABC D curve.
When electrons are emitted, the cathode becomes positive charge and therefore holds electrons near itself. In the absence of an electric field between the cathode and the anode, the emitted electrons form an electron cloud at the cathode.
As the voltage between the anode and cathode increases, more electrons flow to the anode, and therefore the current increases. This dependence is expressed by the section of the OAB graph. Section AB characterizes the direct dependence of current on voltage, i.e. in the voltage range U 1 - U 2, Ohm's law is satisfied.

The nonlinear dependence in section BC D is explained by the fact that the number of electrons rushing to the anode becomes greater than the number of electrons escaping from the cathode.
When enough great importance voltage U 3 all electrons emitted from the cathode reach the anode, and the electric current reaches saturation.
You can also use it as a source of charged particles. radioactive drug emitting α-particles. Under the influence of electric field forces, α-particles will move, i.e. an electric current will occur.
Thus, an electric current in a vacuum can be created by the ordered movement of any charged particles (electrons, ions).
Electric current in semiconductors.
Semiconductors are substances whose resistivity decreases with increasing temperature and depends on the presence of impurities and changes in illumination. The resistivity of conductors at room temperature is in the range from 10 -3 to 10 7 Ohm m. Typical representatives of semiconductors are germanium and silicon crystals.
In these crystals, the atoms are connected to each other by a covalent bond. When heated covalent bond is disrupted, the atoms become ionized. This causes the appearance of free electrons and “holes” - vacant positive places with a missing electron.

In this case, electrons of neighboring atoms can occupy vacant positions, forming a “hole” in the neighboring atom. Thus, not only electrons, but also “holes” can move around the crystal. When such a crystal is placed in an electric field, electrons and holes will come into ordered motion - an electric current will arise.
In a pure crystal, an electric current is created by an equal number of electrons and "holes". Conductivity caused by the movement of free electrons and an equal number of “holes” in a semiconductor crystal without impurities is called intrinsic conductivity of the semiconductor .
As the temperature increases, the intrinsic conductivity of the semiconductor increases, because the number of free electrons and “holes” increases.
The conductivity of conductors depends on the presence of impurities. There are donor and acceptor impurities. Donor impurity- an impurity with a higher valence. For example, for tetravalent silicon the donor impurity is pentavalent arsenic. Four valence electron arsenic atoms participate in the creation of a covalent bond, and the fifth will become a conduction electron.

When heated, the covalent bond is broken, and additional conduction electrons and “holes” appear. Therefore, in a crystal the number of free electrons prevails over the number of “holes”. The conductivity of such a conductor is electronic; a semiconductor is n-type semiconductor. Electrons are main carriers charge, “holes” - non-core .
Acceptor admixture- an impurity with a lower valency. For example, for tetravalent silicon, the acceptor impurity is trivalent indium. Three valence electrons of the indium atom are involved in creating a covalent bond with three silicon atoms, and a “hole” is formed in place of the fourth incomplete covalent bond.

When heated, the covalent bond is broken, and additional conduction electrons and “holes” appear. Therefore, in a crystal the number of “holes” prevails over the number of free electrons. The conductivity of such a conductor is hole, the semiconductor is p-type semiconductor. "Holes" are main carriers charge, electrons - non-core .
When p-type and n-type semiconductors come into contact across the boundary, electrons diffuse from the n-region to the p-region and “holes” from the p-region to the n-region. This results in the formation of a barrier layer that prevents further diffusion. The p-n junction has one-way conductivity.

At p-n connection transition to the current source so that the p-region is connected to the positive pole, and the n-region to the negative pole, the movement of the main charge carriers through the contact layer appears. This connection method is called forward connection.

When a p-n junction is connected to a current source so that the p-region is connected to the negative pole and the n-region to the positive pole, the thickness of the blocking layer increases and the movement of the majority charge carriers through the contact layer stops, but movement of minority charges can occur through the contact layer. This connection method is called reverse connection.

The principle of operation of a semiconductor diode is based on the property of one-way conductivity of the p-n junction. The main application of a semiconductor diode is a current rectifier.

Current-voltage characteristic of a semiconductor diode.
The dependence of current on voltage is expressed by the AOB curve.

The OB branch corresponds to the passing direction of the current, when the current is created by the main charge carriers, and as the voltage increases, the current strength increases. The AO branch corresponds to the current created by minority charge carriers, and the current values are small.
There are no absolute dielectrics in nature. The ordered movement of particles - carriers of electric charge - that is, current, can be caused in any environment, but this requires special conditions. We will look here at how electrical phenomena in gases and how a gas can be transformed from a very good dielectric into a very good conductor. We will be interested in the conditions under which electric current in gases occurs, as well as in what features it is characterized.
Electrical properties of gases
A dielectric is a substance (medium) in which the concentration of particles - free electric charge carriers - does not reach any significant value, as a result of which the conductivity is negligible. All gases are good dielectrics. Their insulating properties are used everywhere. For example, in any switch, the circuit opens when the contacts are brought into such a position that an air gap is formed between them. Wires in power lines are also insulated from each other by an air layer.
The structural unit of any gas is a molecule. It consists of atomic nuclei and electron clouds, that is, it is a collection of electrical charges distributed in some way in space. Due to the peculiarities of its structure, a gas molecule can be polarized under the influence of an external electric field. The vast majority of the molecules that make up a gas are electrically neutral under normal conditions, since the charges in them cancel each other out.
If an electric field is applied to a gas, the molecules will take on a dipole orientation, occupying a spatial position that compensates for the effect of the field. The charged particles present in the gas, under the influence of Coulomb forces, will begin to move: positive ions - towards the cathode, negative ions and electrons - towards the anode. However, if the field has insufficient potential, a single directed flow of charges does not arise, and one can rather speak of individual currents, so weak that they should be neglected. Gas behaves like a dielectric.
Thus, for the occurrence of electric current in gases, a high concentration of free charge carriers and the presence of a field are required.
Ionization
The process of an avalanche-like increase in the number of free charges in a gas is called ionization. Accordingly, the gas in which there is significant amount charged particles is called ionized. It is in such gases that an electric current is created.
The ionization process is associated with a violation of the neutrality of molecules. Due to the removal of an electron, positive ions arise; the addition of an electron to a molecule leads to the formation negative ion. In addition, ionized gas contains many free electrons. Positive ions and especially electrons are the main charge carriers during electric current in gases.
Ionization occurs when a certain amount of energy is imparted to a particle. Thus, the outer electron in the molecule, having received this energy, can leave the molecule. Mutual collisions of charged particles with neutral ones lead to the knocking out of new electrons, and the process takes on an avalanche-like character. The kinetic energy of the particles also increases, which greatly promotes ionization.
Where does the energy expended to excite electric current in gases come from? Ionization of gases has several energy sources, according to which its types are usually named.
- Ionization by electric field. In this case potential energy fields are converted into kinetic energy of particles.
- Thermal ionization. An increase in temperature also leads to the formation large quantity free charges.
- Photoionization. The essence this process is that quanta impart energy to electrons electromagnetic radiation- photons, if they have a sufficiently high frequency (ultraviolet, x-rays, gamma quanta).
- Impact ionization results from the conversion of the kinetic energy of colliding particles into the energy of electron separation. Along with thermal ionization, it serves as the main factor in the excitation of electric current in gases.
Each gas is characterized by a certain threshold value - the ionization energy necessary for an electron to break away from the molecule, overcoming the potential barrier. This value for the first electron ranges from several volts to two tens of volts; To remove the next electron from a molecule, more energy is needed, and so on.
It should be taken into account that simultaneously with ionization in the gas, the reverse process occurs - recombination, that is, the restoration of neutral molecules under the influence of Coulomb attractive forces.
Gas discharge and its types
So, the electric current in gases is caused by the ordered movement of charged particles under the influence of an electric field applied to them. The presence of such charges, in turn, is possible due to various ionization factors.

Thus, thermal ionization requires significant temperatures, but an open flame due to some chemical processes promotes ionization. Even at a relatively low temperature in the presence of a flame, the appearance of an electric current in gases is recorded, and experiment with gas conductivity makes it easy to verify this. It is necessary to place the flame of a burner or candle between the plates of a charged capacitor. The circuit that was previously open due to the air gap in the capacitor will close. A galvanometer connected to the circuit will indicate the presence of current.
Electric current in gases is called gas discharge. It must be borne in mind that in order to maintain discharge stability, the action of the ionizer must be constant, since due to constant recombination the gas loses its electrically conductive properties. Some carriers of electric current in gases - ions - are neutralized at the electrodes, others - electrons - when they reach the anode, they are directed to the “plus” of the field source. If the ionizing factor ceases to act, the gas will immediately become a dielectric again and the current will stop. Such a current, dependent on the action of an external ionizer, is called a non-self-sustaining discharge.
The peculiarities of the passage of electric current through gases are described by a special dependence of the current on voltage - the current-voltage characteristic.

Let us consider the development of a gas discharge on the graph of the current-voltage dependence. When the voltage increases to a certain value U 1, the current increases in proportion to it, that is, Ohm's law is satisfied. The kinetic energy increases, and therefore the speed of charges in the gas, and this process outstrips recombination. At voltage values from U 1 to U 2, this relationship is violated; when U2 is reached, all charge carriers reach the electrodes without having time to recombine. All free charges are used, and a further increase in voltage does not lead to an increase in current. This type of movement of charges is called saturation current. Thus, we can say that the electric current in gases is also due to the peculiarities of the behavior of ionized gas in electric fields of various strengths.
When the potential difference across the electrodes reaches a certain value U 3 , the voltage becomes sufficient for the electric field to cause an avalanche-like ionization of the gas. The kinetic energy of free electrons is already enough for impact ionization of molecules. Their speed in most gases is about 2000 km/s and higher (it is calculated using the approximate formula v=600 Ui, where Ui is the ionization potential). At this moment, gas breakdown occurs and a significant increase in current due to internal source ionization. Therefore, such a discharge is called independent.
The presence of an external ionizer in this case no longer plays a role in maintaining an electric current in the gases. Independent discharge in different conditions and with different characteristics of the electric field source, it may have certain features. There are such types of self-discharge as glow, spark, arc and corona. We will look at how electric current behaves in gases, briefly for each of these types.
A potential difference of 100 (or even less) to 1000 volts is sufficient to initiate a self-discharge. Therefore, a glow discharge, characterized by a low current value (from 10 -5 A to 1 A), occurs at pressures of no more than a few millimeters of mercury.
In a tube with rarefied gas and cold electrodes, the glow discharge that forms looks like a thin glowing cord between the electrodes. If you continue pumping gas from the tube, the cord will be washed out, and at pressures of tenths of a millimeter of mercury, the glow fills the tube almost completely. There is no glow near the cathode - in the so-called dark cathode space. The rest is called the positive column. In this case, the main processes ensuring the existence of the discharge are localized precisely in the dark cathode space and in the area adjacent to it. Here, charged gas particles are accelerated, knocking electrons out of the cathode.

In a glow discharge, the cause of ionization is electron emission from the cathode. Electrons emitted by the cathode produce impact ionization of gas molecules, the resulting positive ions cause secondary emission from the cathode, and so on. The glow of a positive column is mainly due to the release of photons by excited gas molecules, and different gases are characterized by a glow of a certain color. The positive column takes part in the formation of the glow discharge only as a section electrical circuit. If you bring the electrodes closer, you can make the positive column disappear, but the discharge will not stop. However, with a further reduction in the distance between the electrodes, the glow discharge cannot exist.
It should be noted that for of this type electric current in gases, the physics of some processes has not yet been fully clarified. For example, the nature of the forces that cause an expansion of the region on the cathode surface that takes part in the discharge as the current increases remains unclear.
Spark discharge
Spark breakdown has a pulsed nature. It occurs at pressures close to normal atmospheric pressure, in cases where the power of the electric field source is insufficient to maintain a stationary discharge. The field strength is high and can reach 3 MV/m. The phenomenon is characterized sharp increase discharge electric current in the gas, at the same time the voltage drops extremely quickly and the discharge stops. Then the potential difference increases again, and the whole process repeats.
With this type of discharge, short-term spark channels are formed, the growth of which can begin from any point between the electrodes. This is due to the fact that impact ionization occurs randomly in places where it is currently concentrated greatest number ions. Near the spark channel, the gas quickly heats up and experiences thermal expansion, causing acoustic waves. Therefore, a spark discharge is accompanied by a crackling sound, as well as the release of heat and a bright glow. Avalanche ionization processes generate in the spark channel high pressure and temperatures up to 10 thousand degrees and above.
The most striking example of a natural spark discharge is lightning. The diameter of the main lightning spark channel can range from a few centimeters to 4 m, and the length of the channel can reach 10 km. The current strength reaches 500 thousand amperes, and the potential difference between a thundercloud and the surface of the Earth reaches a billion volts.
The longest lightning strike, 321 km long, was observed in 2007 in Oklahoma, USA. The record holder for the longest duration was lightning recorded in 2012 in the French Alps - it lasted over 7.7 seconds. When struck by lightning, the air can heat up to 30 thousand degrees, which is 6 times higher than the temperature of the visible surface of the Sun.
In cases where the power of the electric field source is sufficiently high, the spark discharge develops into an arc discharge.
This type of self-discharge is characterized by a high current density and low (less than a glow discharge) voltage. The breakdown distance is short due to the close proximity of the electrodes. The discharge is initiated by the emission of an electron from the cathode surface (for metal atoms the ionization potential is small compared to gas molecules). During a breakdown, conditions are created between the electrodes under which the gas conducts electric current, and a spark discharge occurs, closing the circuit. If the power of the voltage source is high enough, the spark discharges turn into a stable electric arc.

Ionization during an arc discharge reaches almost 100%, the current is very high and can range from 10 to 100 amperes. At atmospheric pressure, the arc can heat up to 5-6 thousand degrees, and the cathode - up to 3 thousand degrees, which leads to intense thermionic emission from its surface. Bombardment of the anode with electrons leads to partial destruction: a depression is formed on it - a crater with a temperature of about 4000 °C. An increase in pressure entails an even greater increase in temperatures.
When the electrodes are separated, the arc discharge remains stable up to a certain distance, which makes it possible to combat it in those areas of electrical equipment where it is harmful due to the corrosion and burnout of contacts it causes. These are devices such as high-voltage and circuit breakers, contactors and others. One of the methods of combating arcs that occur when contacts open is the use of arc suppression chambers based on the principle of arc elongation. Many other methods are also used: bypassing contacts, using materials with high ionization potential, and so on.
The development of a corona discharge occurs at normal atmospheric pressure in sharply inhomogeneous fields for electrodes with large surface curvature. These could be spiers, masts, wires, various elements electrical equipment having complex shape, and even human hair. Such an electrode is called corona electrode. Ionization processes and, accordingly, gas glow take place only near it.
A corona can form both on the cathode (negative corona) when it is bombarded with ions, and on the anode (positive corona) as a result of photoionization. The negative corona, in which the ionization process as a consequence of thermal emission is directed away from the electrode, is characterized by an even glow. In the positive corona, streamers can be observed - luminous lines of a broken configuration that can turn into spark channels.
An example of a corona discharge in natural conditions are occurring on the tips of tall masts, treetops, and so on. They are formed at high electric field strength in the atmosphere, often before a thunderstorm or during a blizzard. In addition, they were recorded on the skin of aircraft caught in a cloud. volcanic ash.

Corona discharge on power line wires leads to significant losses of electricity. At high voltages, a corona discharge can turn into an arc discharge. They are fighting him in various ways, for example, by increasing the radius of curvature of the conductors.
Electric current in gases and plasma
A fully or partially ionized gas is called plasma and is considered the fourth state of aggregation substances. In general, plasma is electrically neutral, since the total charge of its constituent particles is zero. This distinguishes it from other charged particle systems, such as electron beams.
Under natural conditions, plasma is formed, as a rule, at high temperatures due to the collision of gas atoms at high speeds. The overwhelming majority of baryonic matter in the Universe is in the state of plasma. These are stars, part of the interstellar matter, intergalactic gas. The earth's ionosphere is also a rarefied, weakly ionized plasma.
The degree of ionization is an important characteristic of plasma - its conducting properties depend on it. The degree of ionization is defined as the ratio of the number of ionized atoms to total number atoms per unit volume. The more ionized the plasma, the higher its electrical conductivity. In addition, it is characterized by high mobility.
We see, therefore, that gases that conduct electric current within the discharge channel are nothing more than plasma. Thus, glow and corona discharges are examples of cold plasma; a lightning spark channel or an electric arc are examples of hot, almost completely ionized plasma.
Electric current in metals, liquids and gases - differences and similarities
Let us consider the features that characterize a gas discharge in comparison with the properties of current in other media.
In metals, current is the directed movement of free electrons, which does not entail chemical changes. Conductors of this type are called conductors of the first kind; These include, in addition to metals and alloys, coal, some salts and oxides. They are distinguished by electronic conductivity.
Conductors of the second type are electrolytes, that is, liquid aqueous solutions of alkalis, acids and salts. The passage of current is associated with chemical change electrolyte - electrolysis. Ions of a substance dissolved in water, under the influence of a potential difference, move into opposite sides: positive cations - to the cathode, negative anions - to the anode. The process is accompanied by the release of gas or the deposition of a metal layer on the cathode. Conductors of the second type are characterized by ionic conductivity.
As for the conductivity of gases, it is, firstly, temporary, and secondly, it has signs of similarity and difference with each of them. Thus, electric current in both electrolytes and gases is a drift of oppositely charged particles directed towards opposite electrodes. However, while electrolytes are characterized by purely ionic conductivity, in a gas discharge, with a combination of electronic and ionic types of conductivity, the leading role belongs to electrons. Another difference between electric current in liquids and gases is the nature of ionization. In an electrolyte, the molecules of a dissolved compound dissociate in water, but in a gas, the molecules do not collapse, but only lose electrons. Therefore, a gas discharge, like a current in metals, is not associated with chemical changes.
The current in liquids and gases is also different. The conductivity of electrolytes generally obeys Ohm's law, but during a gas discharge it is not observed. The current-voltage characteristic of gases has much more complex character, associated with the properties of plasma.
It is worth mentioning the general and distinctive features electric current in gases and in vacuum. Vacuum is an almost perfect dielectric. “Almost” - because in a vacuum, despite the absence (more precisely, an extremely low concentration) of free charge carriers, a current is also possible. But potential carriers are already present in the gas; they just need to be ionized. Charge carriers are introduced into the vacuum from the substance. As a rule, this occurs through the process of electron emission, for example when the cathode is heated (thermionic emission). But in various types of gas discharges, emission, as we have seen, plays a role important role.
Application of gas discharges in technology
ABOUT harmful effects certain categories have already been briefly discussed above. Now let's pay attention to the benefits they bring in industry and in everyday life.

Glow discharge is used in electrical engineering (voltage stabilizers) and in coating technology (cathode sputtering method, based on the phenomenon of cathode corrosion). In electronics it is used to produce ion and electron beams. Widely known areas of application of glow discharge are fluorescent and so-called energy-efficient lamps and decorative neon and argon gas discharge tubes. In addition, glow discharge is used in spectroscopy.
Spark discharge is used in fuses and in electrical discharge methods for precision metal processing (spark cutting, drilling, etc.). But it is best known for its use in engine spark plugs. internal combustion and in household appliances (gas stoves).
The arc discharge, having been first used in lighting technology back in 1876 (Yablochkov candle - “Russian light”), still serves as a light source - for example, in projection devices and powerful searchlights. In electrical engineering, the arc is used in mercury rectifiers. In addition, it is used in electric welding, metal cutting, and industrial electric furnaces for smelting steel and alloys.
Corona discharge is used in electric precipitators for ion purification of gases, in meters elementary particles, in lightning rods, in air conditioning systems. Corona discharge also works in photocopiers and laser printers, where it charges and discharges a photosensitive drum and transfers powder from the drum to paper.
Thus, gas discharges of all types find the widest application. Electric current in gases is successfully and effectively used in many fields of technology.