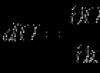2 1. Introduction 1.1. The subject of atomic physics, its brief history of development, goals and objectives 1.2. Basic definitions. Electron, proton, neutron, atom, ion, molecule, nuclide, atomic nucleus, chemical element, isotopes 1.3. Nuclear and shell properties of the atom 1.4. Units of measurement of physical quantities in atomic physics. Electron-volt. Mole, Avogadro's constant, atomic mass unit, relative atomic mass. Scales of energies, lengths, frequencies, masses in atomic and nuclear physics 1.5. Classical, relativistic and quantum physics. Momentum and energy 1.6. Photon. Photon energy scale (electromagnetic radiation scale)

3 Atomic physics Atomic physics (physics of the atom and atomic phenomena) is a branch of physics that studies the structure and properties of atoms, as well as elementary processes in which atoms take part. The objects of study of atomic physics are both atoms and molecules, atomic and molecular ions, exotic atoms and other microparticles In the phenomena studied within the framework of atomic physics, the main role is played by electromagnetic interactions. The results of research in the field of atomic physics serve as the basis for understanding chemical bonds, optical and tunneling phenomena, processes in plasma, neutral liquids, solids (including semiconductors and nanomaterials) Theoretical basis atomic physics itself are quantum theory and quantum electrodynamics. There is no clear boundary between atomic physics and other branches of physics, and in accordance with international classification, atomic physics is included in the field of atomic, molecular physics and optics

4 Brief history development of atomic physics The concept of “atom” was used by ancient Greek scientists (V – II centuries BC) to designate the smallest, indivisible particles, of which everything that exists in the world consists Experimental confirmation of atomistic concepts was obtained in the 19th century in chemical and physical research. The idea that the atom consists of positively and negatively charged parts was substantiated in the second half of the 19th century. In 1897, J. .J. Thomson discovered the electron, and it was soon proven that it is an integral part of all atoms. The idea of an atom as a system consisting of an atomic nucleus and an electron shell was substantiated by E. Rutherford in 1911. After this idea became generally accepted, from the atomic physics, nuclear physics and, somewhat later, physics emerged elementary particles

5 Brief history of the development of atomic physics The foundations of modern atomic physics were laid at the beginning of the 20th century, when, based on the model of the atom by E. Rutherford and the development of quantum concepts of M. Planck (1900) and A. Einstein (1905), N. Bohr gave explanations row the most important properties atom (1913) and two “quantum” postulates were put forward. According to the first of them, there are special (stationary) states of the atom in which the latter does not emit energy, although the charged particles (electrons) included in its composition undergo accelerated motion. According to the second postulate, the radiation of the atom occurs during the transition from one stationary state to another, and the frequency ν of this radiation is determined from the condition h = E – E (Bohr’s frequency rule), where h is Planck’s constant, E and E are the values of the atomic energy in the initial and final states. The first postulate reflects the fact of stability atom, second frequency discreteness in atomic spectra

6 Brief history of the development of atomic physics Bohr's theory, which was unable to comprehensively explain the properties of atoms and molecules, was replaced by a consistent quantum theory created in the 20s - 30s of the 20th century (W. Heisenberg, E. Schrödinger, P. Dirac) Nevertheless, Bohr's postulates still retain their significance and are integrally included in the foundations of the physics of microscopic phenomena. Within the framework of modern quantum theory maximum given full explanation properties of the atom: principles of formation of optical and x-ray spectra, behavior of atoms in magnetic (Zeeman effect) and electric (Stark effect) fields, obtained theoretical basis periodic table of elements and the nature of chemical bonds, calculation methods were developed electronic structure atoms, molecules and solids (Hartree-Fock self-consistent field method), new devices were created to study the structure and properties of matter (electron microscope) The development of ideas of quantum theory (spin hypothesis, Pauli principle, etc.), in turn, relied on experimental research in the field of atomic physics (line spectra of atoms, photoelectric effect, fine and hyperfine structure of spectral lines, experiments by Frank and Hertz, Davisson and Germer, Stern and Gerlach, the Compton effect, the discovery of deuterium and other isotopes, the Auger effect, etc.)

7 Brief history of the development of atomic physics In the second third of the 20th century, within the framework of atomic physics and on the basis of the ideas of quantum theory, new experimental methods of physical research were developed: electron paramagnetic resonance (EPR), photoelectron spectroscopy (PES), electron impact spectroscopy (EI) , devices for their implementation have been created (maser, laser, etc.) The fundamental principles of quantum theory (interference of quantum states, Lamb shift of levels, etc.) have been directly confirmed experimentally, new methods for calculating the electronic structure of matter have been proposed (density functional theory), new methods have been predicted physical phenomena(superradiation) Methods have been developed for experimental studies of processes occurring with single atoms, ions and electrons held by electric and magnetic fields of a special configuration (atomic and ion “traps”)

8 Brief history of the development of atomic physics New results in the field of atomic physics in the last third of the 20th century - beginning of the XXI centuries are mainly associated with the use of lasers. In scientific practice, methods of laser spectroscopy, including nonlinear, are widely used, on the basis of which it became possible to carry out spectroscopic measurements with single atoms and molecules, determine the characteristics of highly excited states of atoms, and study the dynamics of intra-atomic and intramolecular processes lasting up to several femtoseconds (10–15 s) With the help of lasers, it was possible to carry out and study in detail multiphoton processes of interaction of radiation with atomic systems (multiphoton photoelectric effect, frequency multiplication), as well as cooling of individual atoms to ultra-low temperatures Theoretical research last decades in the field of atomic physics are associated with rapid progress computer technology and are aimed at developing effective methods and means for calculating the electronic structure and properties of multielectron atomic systems, taking into account the energy of electron correlation, relativistic quantum mechanical and quantum electrodynamic corrections

9 Atomic physics Research in the field of atomic physics has found many scientific and practical applications For industrial purposes, to determine the elemental composition of a substance, methods of atomic spectral analysis are used, including EPR, FES and SEM. To solve geological, biological and medical problems, methods of remote and local laser spectral atomic analysis are used; for industrial and technical purposes, laser separation of isotopes is carried out Experimental and theoretical methods atomic physics are used in astrophysics (determining the composition and physical characteristics substances of stars and the interstellar medium, research of Rydberg atoms), metrology ( atomic clock) and other fields of science and technology

10 Goals and objectives of the atomic physics course The main goal of the discipline “Physics of the atom and atomic phenomena” as part of the course general physics, is to form basic knowledge on the physics of microscopic phenomena at the atomic-molecular level and the ability to apply them to solve applied problems To achieve this goal, the following tasks are solved: – analysis of the development of atomistic and the formation of quantum concepts; – study of the most important experimental facts of atomic physics and their relationships; – identifying the specifics of micro-phenomena and the inconsistency of the classical theory to explain them; – learning the basics quantum mechanics and methods for solving quantum mechanical problems; – systematic study and explanation based on quantum theory of the structure and properties of atoms and molecules, their behavior in external fields and in interaction with each other

12 Electron Electron is a stable elementary particle with a negative electric charge. The absolute value of the charge of the electron is equal to the elementary charge q e = –e –1.610 –19 C. Mass of the electron m e = m –31 kg. Spin of the electron is ½. The magnetic moment of the electron is approximately equal in magnitude to the Bohr magneton μ e – μ B – –4 eV/T The symbol e or e is used to denote an electron – Electrons form the electron shells of all atoms and ions The electron has an antiparticle positron (e +)



15 Proton Proton is a stable elementary particle with a positive electric charge The charge of a proton is equal to the elementary charge q p = e –19 C Proton mass m p 1836m e –27 kg Proton spin is ½ Magnetic moment of a proton μ p –8 eV/T The proton is designated by the symbol p or p + A proton has an antiparticle antiproton (p –)

16 Annihilation of an antiproton An antiproton (blue track) collides with a proton in a bubble chamber. As a result, four positive pions (red tracks) and four negative ones (green tracks) are produced. The yellow track belongs to a muon, which is born as a result of pion decay

17 Neutron Neutron is an elementary particle with zero electric charge. The lifetime of a neutron in a free state is approximately 886 s. Neutron mass m n 1839m e –27 kg. Neutron spin is ½ Despite the absence electric charge, the neutron has a magnetic moment μ n – –8 eV/T The neutron is designated by the symbol n or n 0 The neutron has an antiparticle antineutron Protons and neutrons are collectively called nucleons Atomic nuclei consist of protons and neutrons
18 Neutron Since neutrons have no electrical charge, they do not leave tracks in particle detector chambers. However, neutrons can be detected by their interaction with other, charged particles. The colorized image shows the tracks of particles in a cloud chamber filled with a mixture of hydrogen gas, ethyl alcohol and water A beam of neutrons penetrates the chamber from below and causes transmutation of the oxygen and carbon atoms that make up the molecules of ethyl alcohol
19 Atom Atom microparticle consisting of an atomic nucleus and surrounding electrons (electron shell) A positively charged nucleus holds negatively charged electrons by forces of electrical attraction Since the nucleus of an atom consists of protons and neutrons, and at the same time the electric charge of a neutron equal to zero, proton to elementary charge e, electron charge is equal to e, then with the number of electrons in the shell, equal to the number protons in the nucleus, the total electric charge of the atom is zero. The dimensions of the nucleus (~ 10 –15 – 10 –14 m) are extremely small compared to the dimensions of the atom (~ 10 –10 m), however, due to the fact that the mass of the proton (as well as neutron) almost 2 thousand times more mass electron, almost the entire mass of the atom () is concentrated in the nucleus

20 Gold atom Au An image of an individual gold atom was obtained using a transmission electron microscope Magnification by a factor of 35 mm to a size of 35 mm


22 Silicon atoms Si Colorized image of silicon atoms obtained using a transmission electron microscope. The unit cell of the crystal is shown. Bonds between atoms are also visible. Increased by a factor of up to a size of 35 mm.


24 Uranium atoms U Colorized image of uranium atoms obtained using a transmission electron microscope Small dots correct form– individual atoms, larger formations – clusters consisting of 2–20 atoms. The size of the field of view is approximately 100 Å. Increased by a factor of 35mm

25 Uranyl microcrystals UO 2 2+ A colored image of uranyl microcrystals was obtained using a transmission electron microscope. Each spot represents single atom uranium Increased by a factor of 35 mm


27 Chemical element, nuclide, isotopes Atoms with a certain number of protons Z in the nucleus belong to the same chemical element. The number Z is called the atomic number chemical element. A collection of atoms with a certain number of protons Z and neutrons N in the nucleus is called a nuclide. Nuclides are designated by adding the mass number A to the name of the element, equal to the amount Z + N (eg oxygen-16, uranium-235), or by placing the number A next to the element symbol (16 O, 235 U). Nuclides of the same element are called isotopes. The mass of the lightest atom of the hydrogen atom, consisting of one proton and one electron, is equal to m H 1.67·10 –27 kg. The masses of the remaining atoms are approximately A times greater than m H. There are 90 chemical elements and more than 300 different nuclides found in nature; 270 of them are stable, the rest are radioactive. Some radioactive nuclides are obtained artificially.




31 Ions The process of removing or adding electrons to an atom is called ionization. When the number of electrons in the shell is less than Z, a positive atomic ion is obtained; when Z is greater than Z, it is negative. Thus, an ion is an electrically charged atom (or molecule) that is formed by detachment or addition one or more electrons to a neutral atom (or molecule)

32 Ions Positively charged ions are called cations, negatively charged anions. Ions are designated by a chemical symbol with an index that indicates the multiplicity (the amount of charge in units of elementary charge) and the sign of the ion: H –, Na +, UO 2 2+ Ions can be represented as sustainable formations(usually in solutions or crystals) and unstable (in gases under ordinary conditions) Atomic cations can be obtained up to a charge of +(Z – 1). Thus, for example, U 90+ and U 91+ were obtained at ion accelerators. Atomic anions with a charge of 2 or more do not exist in a free state


34 Molecule A molecule is the smallest stable particle of a substance, consisting of more than one atom. A molecule is characterized by a certain composition. atomic nuclei, number of electrons and spatial structure To indicate the quantitative and qualitative composition of molecules, chemical formulas are used: O 2 (oxygen molecule), H 2 O (water molecule), CH 4 (methane molecule), C 6 H 6 (benzene molecule), C 60 (fullerene molecule)




39 DNA molecule A colored image of a DNA molecule was obtained using a transmission electron microscope in a camera with high vacuum the DNA sample is coated with a thin layer of platinum. The metal coating gives a high-contrast image electron microscope

40 Nuclear and shell properties of the atom Nuclear propertiesShell properties Determined by the composition of the nucleus: radioactivity, ability to participate in nuclear reactions, etc. Determined by the structure of the electronic shell: chemical, physical (electrical, magnetic, optical, etc.) 42 Energy Unit of energy in The SI unit is the joule (J), however, for energy quantities of objects and phenomena of atomic physics, such a unit is rarely used. More commonly used is an extra-system unit of energy called the electron-volt (eV, eV). An electron-volt is the energy acquired by a charged particle with an elementary charge, passing through an accelerating potential difference of 1 volt: 1 eV = J To measure energies in atomic and nuclear physics, multiples (keV, 1 keV = 10 3 eV, MeV, 1 MeV = 10 6 eV) and submultiples (μeV, 1 μeV = 10 –6 eV) electron-volt units, as well as some others: Rydberg (Ry), Hartree (Ha, or atomic unit, a.u.) Rydberg numerically equal to energy ionization of a hydrogen atom from the ground state in the approximation of infinite nuclear mass: 1 Ry eV Hartree is equal to the absolute value of the potential energy of an electron in the ground state of a hydrogen atom in the approximation of infinite nuclear mass: 1 Ha = 2 Ry eV The energies of states of atomic systems, as well as transitions between states can measured in other units

43 Mass The SI unit of mass is the kilogram (kg), however, to measure the masses of objects in atomic physics, an extra-systemic unit of measurement is used, called the atomic mass unit (a.m.u.) An atomic mass unit is equal to 1/12 of the mass of an unbound, unexcited carbon-12 atom (12 C): 1 a. e. m kg 1 a. e.m. is approximately equal to the mass of one proton or neutron. Relative atomic mass is the mass of an atom, expressed in a. e.m. Avogadro's constant N A physical constant, numerically equal to the number of atoms in 12 g of the pure isotope carbon-12: N A mol –1 Mole (SI unit of quantity of a substance) by definition contains N A structural elements(atoms, molecules, ions).

44 Length The SI unit of length is the meter (m). 1 meter equal to the distance, which light travels in a vacuum in a time interval equal to 1/ second. With the exception of measurements of wavelengths of electromagnetic radiation in the radio range, such a unit of length is rarely used in atomic physics, and instead, submultiple units of a meter are used to measure linear dimensions, as well as wavelengths: centimeter (cm, 1 cm = 10 –2 m), millimeter ( mm, 1 mm = 10–3 m), micrometer (μm, μm, 1 μm = 10–6 m), nanometer (nm, 1 nm = 10–9 m), picometer (pm, 1 pm = 10–12 m ) and others, as well as extra-systemic units: angstrom (Å, 1 Å = 0.1 nm = 10 –10 m), boron (or Bohr radius) (1 bohr Å)

45 Time The unit of duration of time intervals in SI is the second (s). Currently, the second is determined on the basis of the so-called. atomic time standard: one second (or atomic second) is equal to the periods of electromagnetic radiation corresponding to the energy transition between two levels of the hyperfine structure of the ground state of the isotope 133 Cs (cesium-133). The duration of fast processes in atomic physics is usually measured in sub-second units: nano-, pico- or femtoseconds (ns, ps, fs, 1 fs = 10 –15 s)

46 Scales of physical quantities in atomic and nuclear physics The phenomena of atomic physics are characterized by sizes from 10–12 m (inner subshells of heavy atoms) to tenths of a nanometer (sizes of atoms and small molecules), energies from 10–6 eV (ultrafine structure of levels) to 10 5 eV (binding energy of inner subshell electrons), times from tens of femtoseconds (duration of ultrashort laser pulses) to thousands of seconds (lifetime of metastable states of atoms) Typical molecular sizes are 0.1–1 nm. The internuclear distance for the smallest molecule (H 2) is equal to nm. Macromolecules of DNA and many polymers can have macroscopic dimensions. Thus, the length of an unfolded DNA helix can reach several centimeters with a width of approximately 2 nm.

47 Photon Photon, or quantum of electromagnetic radiation (field), a massless elementary particle that has no electric charge. In a vacuum, a photon moves with a speed c. A photon has a spin equal to 1. Projections of the spin onto the directions, perpendicular to the direction propagation of a photon, determine the state of its polarization. The photon is designated by the symbol γ
Atomic physics arose at the turn of the 19th and 20th centuries based on studies of the optical spectra of gases, the discovery of the electron and radioactivity. At the first stage of its development (the first quarter of the 20th century), atomic physics was mainly concerned with identifying the structure of the atom and studying its properties. E. Rutherford's experiments on the scattering of alpha particles by thin metal foil (1908-1911) led to the creation of a planetary model of the atom; Using this model, N. Bohr (1913) and A. Sommerfeld (1915) developed the first quantitative theory of the atom (see Atom). Subsequent studies of the properties of the electron and atoms culminated in the creation in the mid-20s. quantum mechanics - physical theory, which describes the laws of the microworld and allows quantitative consideration of phenomena in which microparticles participate (see Quantum mechanics).
Quantum mechanics is the theoretical foundation of atomic physics. At the same time, atomic physics plays the role of a kind of “ test site" for quantum mechanics. Concepts and conclusions of quantum mechanics, often inconsistent with ours everyday experience, undergo experimental testing in atomic physics. A striking example The famous experiments of Frank-Hertz (1913) and Stern-Gerlach (1922) can serve as an example; Let's look at them in more detail below.
By the beginning of the 20th century. Rich material has been accumulated on the optical spectra of atoms. It was found that each chemical element has its own line spectrum, characterized by a regular, ordered arrangement of spectral lines. Quantum mechanics connects the observed patterns in the spectrum with the system of energy levels of a given atom. In 1913, German physicists J. Frank and G. Hertz performed an experiment that gave direct experimental confirmation that internal energy the atom is quantized and therefore can only change discretely, that is, in certain portions. They measured the energy of free electrons expended to excite mercury atoms. The main element of the installation is an evacuated glass cylinder with three soldered electrodes: a cathode, an anode, and a grid (the prototype of a modern vacuum triode). The cylinder contained mercury vapor under a pressure of 1 mmHg. Art. The electrons that left the cathode were accelerated in the field between the cathode and the grid (accelerating voltage U) and then decelerated in the field between the grid and the anode (braking voltage U 1). On the way from the cathode to the anode, the electrons collided with mercury atoms. The voltage U 1 was chosen to be significantly less than U\; therefore, only sufficiently slow electrons were repelled from the anode - those that had lost energy) as a result of inelastic collisions with mercury atoms. In the experiment, the strength of the anode current was measured depending on the accelerating voltage U. The experimental curve has a number of clear maxima, spaced 4.9 V from each other. The appearance of this curve is explained as follows. At U< 4,9 В столкновения электронов с атомами ртути являются упругими (возбуждение атомов не происходит), поэтому сила тока плавно нарастает с увеличением U. По достижении значения U = 4,9 В начинаются неупругие столкновения, связанные с возбуждением атомов ртути; в результате сила тока резко падает. При дальнейшем повышении U ток снова нарастает до тех пор, пока напряжение не достигнет 9,8 В, когда электрон оказывается в состоянии возбудить два атома. При достижении 14,7 В электроны способны испытать три неупругих столкновения с атомами ртути и т. д. При напряжении 4,9 В электрон приобретает энергию 4,9 эВ. Таким образом вид кривой 1(10 показывает, что для возбуждения атома ртути необходима энергия, равная 4,9 эВ. Это и есть, очевидно, та самая порция энергии, которой атом ртути обменивается с электроном.
With a more careful setup of experiments of this type, it was possible to detect the excitation of the following energy levels of atoms: for mercury it is 6.7; 8.3 eV, etc. (10.4 eV is the ionization potential). Observation of the gas glow shows the appearance of a full spectrum for mercury atoms.
An electron moving around an atomic nucleus can be likened to an elementary electric current; it generates a magnetic field. Magnetic fields different electrons, adding up, form the magnetic field of the atom. To characterize it we introduce vector quantity, called the magnetic moment. If electrons completely fill one or another shell (1s, 2s, 2p, etc.), then their magnetic fields cancel each other out; the magnetic moments of the corresponding atoms are zero.
In 1922 in Germany, O. Stern and W. Gerlach performed an experiment that showed that magnetic moment the atom is spatially quantized. They sent a beam of atoms having a magnetic moment through a non-uniform magnetic field and studied the deflections of the atoms under the influence of this field. The degree and nature of the deviation depend on the orientation of the magnetic moment of the atom relative to the direction of the field. If the beam contained atoms with all possible orientations of magnetic moments, then a continuous angular “blurring” of the original beam would be observed. Experimentally, a clear splitting of a beam of atoms into several beams was observed; this meant that the magnetic moment of the atom is spatially quantized - its projection onto the direction of the magnetic field can have only certain specific (discrete) values.
Let us turn to the distribution of deviations of sodium atoms in a nonuniform magnetic field (obtained in 1930). This distribution has two clear maxima. The sodium atom has three filled shells (1s, 2s, 2p) and one 3s electron. The electron cloud of s-electrons is spherically symmetrical (see Atom), therefore their movement in the field of the nucleus does not lead to the appearance of a magnetic moment. To explain the observed splitting of a beam of sodium atoms into two components, it is necessary to assume that the electron has its own magnetic moment, which is not associated with the movement of the electron around the nucleus. This magnetic moment is conventionally associated with the rotation of the electron around its own axis and is called the spin moment (see Spin). The magnetic moment of the electron, associated with its motion around the nucleus, is called the orbital moment. So, in the case of the sodium atom, both the orbital and spin moments of the electrons of the filled shells are mutually compensated; the orbital moment of the 3s electron is zero, and the spin moment of this electron causes the splitting of a beam of sodium atoms in a nonuniform magnetic field. The fact that splitting into two beams is observed means that the spin moment of the electron has two projections onto the direction of the magnetic field.
In the 30s our century has begun new stage in the development of atomic physics. During these years, it became clear that the nature of the interactions responsible for the processes inside the atomic nucleus and explaining the stability or radioactivity of nuclei is completely different compared to the interactions that determine the processes occurring in the electronic shells of the atom (see Unity of the forces of nature). In this regard, a separate scientific direction emerged from atomic physics, related to research into the physics of atomic nuclei; in the 40s This direction took shape into an independent physical science - nuclear physics. Finally, in the 50s. From nuclear physics, a direction related to the study of the systematics and interconversions of elementary particles sprang up - elementary particle physics.
In the end it was completely revealed certain circle issues that make up the content of modern atomic physics. She is not interested in the processes occurring in the atomic nucleus, as well as the interconversions of elementary particles. Atomic physics studies processes involving atoms or ions, and only those processes that do not lead to any changes in atomic nuclei. Consequently, we are talking about processes that affect only the electronic shells of atoms. To similar
processes include: changes in the states of electrons in an atom under the influence of external electric or magnetic fields (for example, under the influence of external fields, the energy levels of atoms are split); absorption and emission of electromagnetic radiation by atoms (see Spectroscopy, X-rays, Photoelectric effect, Lasers); collisions of atoms with free electrons, as well as with other atoms, ions, molecules (as a result of collisions with electrons or other micro-objects, atoms can be excited, transition from an excited state to a less excited state, turn into ions, see Electric discharge in gases); interactions between the electronic shells of various atoms, leading to the formation of molecules and crystals. All these processes are caused electromagnetic interaction. The probabilities of these processes are calculated using the apparatus of quantum mechanics.
Modern atomic physics also studies a special type of atoms called mesoatoms. A mesoatom arises from an ordinary atom as a result of replacing one of the electrons with a muon (μ-), antimeson (π-, K-), an antiproton, or a negatively charged hyperon (see Hadrons, Leptons). There are also anomalous “hydrogen” atoms - positronium, muonium, in which the role of a proton is played by positrons or positively charged antimuons (μ+). All these atoms are unstable; their lifetime is limited by the lifetime of the above particles or the processes of e+ e- and pp-annihilation. Mesoatoms are formed during the process of particle deceleration - as a result of the capture of negatively charged particles by the Coulomb field of atomic nuclei or during capture by positrons and antimuons atomic electrons. Experiments with various anomalous atoms are of great interest both for studying the properties of matter and for studying nuclei and elementary particles.
The special theory of relativity (SRT) is based on two postulates:
- Principle of relativity: in any inertial reference frames, all physical phenomena under the same initial conditions proceed in the same way, i.e. No experiments carried out in a closed system of bodies can determine whether the body is at rest or moving uniformly and in a straight line.
- The principle of the constancy of the speed of light: in all inertial reference systems, the speed of light in vacuum is the same and does not depend on the speed of the moving light source.
Equally important with the postulates of SRT is the position of SRT on the limiting nature of the speed of light in a vacuum: the speed of any signal in nature cannot exceed the speed of light in a vacuum: c= 3∙10 8 m/s. When objects move at a speed comparable to the speed of light, various effects are observed, described below.
1. Relativistic length contraction.
The length of a body in the frame of reference where it is at rest is called its own length L 0 . Then the length of a body moving with speed V in the inertial reference frame decreases in the direction of motion to the length:
Where: c– speed of light in vacuum, L 0 – body length in a fixed frame of reference (length of a body at rest), L– length of the body in the reference frame moving with speed V(length of a body moving at speed V). Thus, body length is relative. The contraction of bodies is noticeable only at speeds comparable to the speed of light.
2. Relativistic extension time of the event.
The duration of a phenomenon occurring at a certain point in space will be the shortest in the inertial frame of reference relative to which this point is motionless. This means that clocks moving relative to an inertial reference frame run slower than stationary clocks and show a longer time interval between events. Relativistic time dilation becomes noticeable only at speeds comparable to the speed of light, and is expressed by the formula:

Time τ 0, measured from a clock at rest relative to the body, is called the proper time of the event.
3. Relativistic law of addition of velocities.
The law of addition of velocities in Newtonian mechanics contradicts the postulates of the SRT and is replaced by a new one relativistic law addition of speeds. If two bodies are moving towards each other, then their speed of approach is expressed by the formula:

Where: V 1 and V 2 – the speed of movement of bodies relative to a fixed frame of reference. If bodies move in the same direction, then their relative speed is:

4. Relativistic increase in mass.
Mass of a moving body m greater than the rest mass of the body m 0:

5. Relationship between energy and body weight.
From the point of view of the theory of relativity, the mass of a body and the energy of a body are practically the same thing. Thus, only the fact of the existence of a body means that the body has energy. Lowest energy E 0 the body has in the inertial frame of reference relative to which it is at rest and is called body's own energy (rest energy of the body):
Any change in body energy means a change in body weight and vice versa:
![]()
where: ∆ E– change in body energy, ∆ m– corresponding change in mass. Total body energy:
Where: m– body weight. Total body energy E proportional relativistic mass and depends on the speed of the moving body, in this sense the following relationships are important:
By the way, the kinetic energy of a body moving at a relativistic speed can only be calculated using the formula:
![]()
From the point of view of the theory of relativity, the law of conservation of rest masses is unfair. For example, the rest mass of an atomic nucleus is less than the sum of the rest masses of the particles included in the nucleus. However, the rest mass of a particle capable of spontaneous decay is greater than the sum own masses its components.
This does not mean a violation of the law of conservation of mass. In the theory of relativity, the law of conservation of relativistic mass is valid, since in isolated system bodies, the total energy is conserved, and therefore the relativistic mass, which follows from Einstein’s formula, thus we can talk about a unified law of conservation of mass and energy. This does not mean the possibility of converting mass into energy and vice versa.
There is a relationship between the total energy of the body, rest energy and momentum:
![]()
Photon and its properties
Light is a stream of quanta of electromagnetic radiation called photons. Photon is a particle that transfers light energy. It cannot be at rest, but always moves at a speed equal speed Sveta. The photon has the following characteristics:
1. Photon energy is equal to:
![]()
Where: h= 6.63∙10 –34 J∙s = 4.14∙10 –15 eV∙s – Planck’s constant, ν – frequency of light, λ – wavelength of light, c– speed of light in vacuum. The energy of a photon in Joules is very small, therefore, for mathematical convenience, it is often measured in an extrasystemic unit - electron volts:
1 eV = 1.6∙10 –19 J.
2. A photon moves in a vacuum at the speed of light c.
3. A photon has momentum:
![]()
4. A photon does not have mass in the usual sense (that mass that can be measured on a scale, calculated using Newton’s second law, and so on), but in accordance with Einstein’s theory of relativity, it has mass as a measure of energy ( E = mc 2). Indeed, any body that has some energy also has mass. If we take into account that a photon has energy, then it also has mass, which can be found as:
![]()
5. A photon has no electrical charge.
Light has a dual nature. As light spreads, it appears wave properties(interference, diffraction, polarization), and when interacting with matter - corpuscular (photoelectric effect). This dual nature of light is called wave-particle duality.
External photoeffect
Photoelectric effect– a phenomenon consisting in the appearance of a photocurrent in a vacuum cylinder when the cathode is illuminated with monochromatic light of a certain wavelength λ .
When the voltage at the anode is negative, the electric field between the cathode and the anode inhibits the electrons. Measuring this holding voltage at which the photocurrent disappears, we can determine the maximum kinetic energy of photoelectrons ejected from the cathode:

Numerous experimenters have established the following basic laws of the photoelectric effect:
- The photoelectric effect is inertialess. This means that electrons begin to fly out of the metal immediately after irradiation with light begins.
- The maximum kinetic energy of photoelectrons increases linearly with increasing light frequency ν and does not depend on its intensity.
- For each substance there is a so-called red photo effect border, that is, the lowest frequency ν min (or greatest length waves λ max) at which the external photoelectric effect is still possible.
- The number of photoelectrons emitted by light from the cathode in 1 s is directly proportional to the light intensity.
When interacting with matter, a photon completely transfers all its energy E = hν one electron. The electron can dissipate part of this energy during collisions with atoms of matter. In addition, part of the electron energy is spent on overcoming the potential barrier at the metal-vacuum interface. To do this, the electron must make work function A out, depending on the properties of the cathode material. The greatest kinetic energy that a photoelectron emitted from the cathode can have, in this case, is determined by the law of conservation of energy:

This formula is usually called Einstein's equation for the external photoelectric effect. Using Einstein's equation, all the laws of the external photoelectric effect can be explained. For red border photo effect, according to Einstein’s formula, we can obtain the expression:

Bohr's postulates
Bohr's first postulate (postulate of stationary states): an atomic system can only be in special stationary or quantum states, each of which corresponds to a specific number n and energy E n. In stationary states, the atom does not emit or absorb energy.
The state with the lowest energy is assigned the number "1". It's called main. All other states are assigned sequential numbers “2”, “3” and so on. They are called excited. An atom can remain in the ground state indefinitely. In the excited state, the atom lives for some time (about 10 ns) and goes into the ground state.
According to Bohr's first postulate, an atom is characterized by a system of energy levels, each of which corresponds to a specific stationary state. The mechanical energy of an electron moving along a closed path around a positively charged nucleus is negative. Therefore, all stationary states correspond to energy values E n < 0. При E n≥ 0 the electron moves away from the nucleus (ionization occurs). Magnitude | E 1 | called ionization energy. State of energy E 1 is called the ground state of the atom.
Bohr's second postulate (frequency rule): when an atom transitions from one stationary state with energy E n to another stationary state with energy E m a quantum is emitted or absorbed, the energy of which is equal to the difference between the energies of stationary states:
![]()
Hydrogen atom
The simplest atom is the hydrogen atom. It contains a single electron. The nucleus of an atom is a proton, a positively charged particle whose charge is equal in magnitude to the charge of an electron. Usually the electron is on the first (ground, unexcited) energy level(an electron, like any other system, tends to a state with a minimum energy). In this state its energy is equal to E 1 = –13.6 eV. In the hydrogen atom, the following relationships are satisfied, connecting the radius of the trajectory of an electron rotating around the nucleus, its speed and energy in the first orbit with similar characteristics in the remaining orbits:
At any orbit in a hydrogen atom, the kinetic ( TO) and potential ( P) electron energies are related to the total energy ( E) by the following formulas:
![]()
![]()
Atomic nucleus
It is now firmly established that atomic nuclei various elements consist of two particles - protons and neutrons, which are commonly called nucleons. A number of notations are introduced to characterize atomic nuclei. The number of protons that make up the atomic nucleus is denoted by the symbol Z and is called the charge number or atomic number (this is an ordinal number in the periodic table). The number of neutrons is denoted by the symbol N. The total number of nucleons (that is, protons and neutrons) is called the mass number A, for which the following formula can be written:
Energy of communication. Mass defect
The most important role in nuclear physics is played by the concept nuclear binding energy. The binding energy of a nucleus is equal to the minimum energy that must be expended to completely split the nucleus into individual particles. From the law of conservation of energy it follows that the binding energy is equal to the energy that is released during the formation of a nucleus from individual particles.
The binding energy of any nucleus can be determined by accurately measuring its mass. Such measurements show that the mass of any nucleus M I is always less than the sum of the masses of the protons and neutrons included in its composition: M I< Zm p+N m n. In this case, the difference between these masses is called mass defect, and is calculated by the formula:
The mass defect can be determined using Einstein's formula E = mc 2 energy released during the formation of a given nucleus, that is, the binding energy of the nucleus E St:
But it is more convenient to calculate the binding energy using another formula (here the masses are taken in atomic units, and the binding energy is obtained in MeV):
![]()
Radioactivity. Law of Radioactive Decay
Almost 90% of known atomic nuclei are unstable. An unstable nucleus spontaneously transforms into other nuclei, emitting particles. This property of nuclei is called radioactivity.
Alpha decay. Alpha decay is the spontaneous transformation of an atomic nucleus with the number of protons Z and neutrons N into another (daughter) nucleus containing the number of protons Z – 2 and neutrons N – 2. In this case, α -particle – the nucleus of a helium atom 4 2 He. General alpha decay scheme:
![]()
Beta decay. During beta decay, an electron is emitted from the nucleus (0–1 e). Beta decay scheme:
![]()
Gamma decay. Unlike α - And β -radioactivity γ -radioactivity of nuclei is not associated with a change in the internal structure of the nucleus and is not accompanied by a change in charge or mass numbers. As in α - and so on β -decay, the daughter nucleus may find itself in some excited state and have an excess of energy. The transition of a nucleus from an excited state to a ground state is accompanied by the emission of one or more γ -quanta, the energy of which can reach several MeV.
Law of radioactive decay. In any sample radioactive substance contains a huge number of radioactive atoms. Since radioactive decay is random and does not depend on external conditions, the law of decreasing quantity N(t) undecayed at this point in time t nuclei can serve as an important statistical characteristic of the radioactive decay process. The law of radioactive decay has the form:
![]()
Magnitude T called half-life, N 0 – starting number radioactive nuclei at t= 0. Half-life is the main quantity characterizing the rate of radioactive decay. The shorter the half-life, the more intense the decay.
At α - And β -radioactive decay, the daughter nucleus may also become unstable. Therefore, a series of successive radioactive decays, which end in the formation of stable nuclei.
Nuclear reactions
nuclear reaction is the process of interaction of an atomic nucleus with another nucleus or elementary particle, accompanied by a change in the composition and structure of the nucleus and the release of secondary particles or γ -quanta. As a result of nuclear reactions, new radioactive isotopes can be formed that are not found on Earth under natural conditions.
In nuclear reactions, several conservation laws are satisfied: momentum, energy, angular momentum, charge. In addition to these classical laws conservation in nuclear reactions is fulfilled law of conservation of the so-called baryon charge(that is, the number of nucleons - protons and neutrons). For example, in a general reaction:
In progress following conditions (total number nucleons before and after the reaction remains unchanged):
![]()
Energy output of a nuclear reaction
Nuclear reactions are accompanied by energy transformations. The energy output of a nuclear reaction is the quantity:
Where: M A and M B – masses starting products, M C and M D – masses of final reaction products. Value Δ M called mass defect. Nuclear reactions can occur with the release of ( Q> 0) or with energy absorption ( Q < 0). Во втором случае первоначальная кинетическая энергия исходных продуктов должна превышать величину |Q|, which is called reaction threshold.
In order for a nuclear reaction to have a positive energy yield, the specific binding energy of nucleons in the nuclei of the initial products must be less specific energy bonds of nucleons in the nuclei of the final products. This means that the value Δ M
Successful, diligent and responsible implementation of these three points will allow you to show up on the CT excellent result, the maximum of what you are capable of.
Found a mistake?
If you think you have found an error in educational materials, then please write about it by email. You can also report an error on the social network (). In the letter, indicate the subject (physics or mathematics), the name or number of the topic or test, the number of the problem, or the place in the text (page) where, in your opinion, there is an error. Also describe what the suspected error is. Your letter will not go unnoticed, the error will either be corrected, or you will be explained why it is not an error.
Atomic physics a branch of physics that studies the structure and state of atoms. A.f. arose in the late 19th - early 20th centuries. In the 10s. 20th century It was found that an atom consists of a nucleus and electrons connected by electrical forces. At the first stage of its development, A. f. also covered issues related to the structure of the atomic nucleus. In the 30s It turned out that the nature of the interactions taking place in the atomic nucleus is different than in the outer shell of the atom, and in the 40s. nuclear physics became an independent field of science. In the 50s Elementary particle physics, or high-energy physics, sprang from it. Prehistory of atomic physics: the doctrine of atoms in the 17th-19th centuries. The idea of the existence of atoms as indivisible particles of matter arose in ancient times; The ideas of atomism were first expressed by the ancient Greek thinkers Democritus and Epicurus. In the 17th century they were revived by the French philosopher P. Gassendi and the English chemist R. Boyle. The ideas about atoms that prevailed in the 17th and 18th centuries were poorly defined. Atoms were considered to be absolutely indivisible and unchanging solid particles, the different types of which differ from each other in size and shape. Combinations of atoms in one order or another form various bodies; the movements of atoms determine all phenomena occurring in matter. I. Newton, M. V. Lomonosov and some other scientists believed that atoms could stick together into more complex particles - “corpuscles”. However, atoms were not assigned specific chemical and physical properties. Atomism still had an abstract, natural-philosophical character. At the end of the 18th - beginning of the 19th centuries. As a result of the rapid development of chemistry, the basis for quantitative development atomic doctrine. The English scientist J. Dalton was the first (1803) to consider an atom as the smallest particle of a chemical element, differing from atoms of other elements in its mass. According to Dalton, the main characteristic of an atom is its atomic mass. Chemical compounds are a collection of “composite atoms” containing certain (characteristic for a given complex substance) number of atoms of each element. All chemical reactions are just rearrangements of atoms into new complex particles. Based on these provisions, Dalton formulated his law of multiple ratios (see Law of multiple ratios). The studies of Italian scientists A. Avogadro (1811) and, in particular, S. Cannizzaro (1858) drew a clear line between an atom and a molecule. In the 19th century along with chemical properties atoms, their optical properties were studied. It was found that each element has a characteristic optical spectrum; spectral analysis was discovered (German physicists G. Kirchhoff and R. Bunsen, 1860). Thus, the atom appeared as a qualitatively unique particle of matter, characterized by strictly defined physical and chemical properties. But the properties of the atom were considered eternal and inexplicable. It was believed that the number of types of atoms (chemical elements) is random and that there is no connection between them. However, it gradually became clear that there are groups of elements that have the same chemical properties - the same maximum valence, and similar laws of change (when moving from one group to another) of physical properties - melting point, compressibility, etc. In 1869, D. I. Mendeleev discovered the periodic table system of elements (See Periodic table of elements). He showed that as the atomic mass of elements increases, their chemical and physical properties repeat periodically ( rice. 1
And 2
). The periodic table proved the existence of bonds between different types of atoms. The conclusion was that the atom has complex structure, changing with atomic mass. The problem of revealing the structure of the atom has become the most important in chemistry and physics (for more details, see Atomism).
The emergence of atomic physics. Major events In science, from which atomic theory originates, there were the discoveries of the electron and radioactivity. When researching the passage electric current through highly rarefied gases, rays were discovered emitted by the cathode of the discharge tube (cathode rays) and having the property of being deflected in the transverse electric and magnetic fields. It turned out that these rays consist of fast-moving negatively charged particles called electrons. In 1897, the English physicist J. J. Thomson measured the charge ratio e of these particles to their mass m. It has also been discovered that metals, when intensely heated or illuminated with short wavelength light, emit electrons (see Thermionic emission, Photoelectron emission). From this it was concluded that electrons are part of any atom. From here it further followed that neutral atoms must also contain positively charged particles. Positively charged atoms - ions - were actually discovered during research electrical discharges in rarefied gases. The idea of an atom as a system of charged particles explained, according to the theory of the Dutch physicist H. Lorentz a ,
the very possibility of an atom emitting light (electromagnetic waves): electromagnetic radiation occurs when intra-atomic charges oscillate; this was confirmed by studying the effect of a magnetic field on atomic spectra (see Zeeman phenomenon). It turned out that the ratio of the charge of intra-atomic electrons to their mass e/m, found by Lorentz in his theory of the Zeeman phenomenon is exactly equal to the value e/m for free electrons, obtained in Thomson's experiments. The theory of electrons and its experimental confirmation provided indisputable proof of the complexity of the atom. The idea of the indivisibility and intransmutability of the atom was finally refuted by the works of the French scientists M. Sklodowska-Curie (See Sklodowska-Curie) and P. Curie (See Curie-Sklodowska) .
As a result of studying radioactivity, it was established (F. Soddy) ,
that atoms undergo two types of transformations. Having emitted an alpha particle (helium ion with a positive charge of 2 e), an atom of a radioactive chemical element turns into an atom of another element located in the periodic table 2 cells to the left, for example, a polonium atom turns into a lead atom. By emitting a beta particle (electron) with a negative charge - e, an atom of a radioactive chemical element turns into an atom of an element located 1 cell to the right, for example, a bismuth atom turns into a polonium atom. The mass of an atom formed as a result of such transformations sometimes turned out to be different from the atomic weight of the element into whose cell it fell. From this it followed the existence of varieties of atoms of the same chemical element with different masses; these varieties were later called isotopes (i.e., occupying the same place in the periodic table). So, the idea of the absolute identity of all atoms of a given chemical element turned out to be incorrect. The results of studying the properties of electrons and radioactivity made it possible to build specific models of the atom. In the model proposed by Thomson in 1903, the atom was represented as a positively charged sphere, in which small (compared to the atom) negative electrons ( rice. 3
). They are held in the atom due to the fact that the attractive forces of their distributed positive charge are balanced by the forces of their mutual repulsion. The Thomson model provided a well-known explanation for the possibility of emission, scattering and absorption of light by an atom. When electrons are displaced from an equilibrium position, an “elastic” force arises, tending to restore equilibrium; this force is proportional to the displacement of the electron from equilibrium position and therefore dipole moment(See dipole moment)
atom. Under the influence of the electrical forces of the incident electromagnetic wave, the electrons in the atom oscillate with the same frequency as electrical intensity in a light wave; The oscillating electrons, in turn, emit light of the same frequency. This is how electromagnetic waves are scattered by atoms of matter. By the degree of attenuation of the light beam in the thickness of the substance, you can find out the total number of scattering electrons, and knowing the number of atoms per unit volume, you can determine the number of electrons in each atom. Rutherford's creation of a planetary model of the atom. Thomson's model of the atom turned out to be unsatisfactory. On its basis, it was not possible to explain the completely unexpected result of the experiments of the English physicist E. Rutherford and his collaborators H. Geiger and E. Marsden on the scattering of alpha particles by atoms. In these experiments, fast α particles were used to directly probe atoms. Passing through matter, alpha particles collide with atoms. With each collision, an α-particle, flying through the electric field of the atom, changes the direction of movement - experiences scattering. In the overwhelming majority of scattering events, the deviations of α-particles (scattering angles) were very small. Therefore, when a beam of α-particles passes through thin layer substance, only a slight blurring of the beam occurred. However, a very small fraction of α particles were deflected at angles greater than 90°. This result could not be explained on the basis of Thomson's model, because the electric field in a “solid” atom is not strong enough to deflect a fast and massive α particle through a large angle. To explain the results of experiments on the scattering of α particles, Rutherford proposed a fundamentally new model of the atom, reminiscent in structure solar system and called planetary. It looks like this: At the center of the atom there is a positively charged nucleus, the dimensions of which (Atomic physics 10 -12 cm) are very small compared to the size of an atom (Atomic physics 10 -8 cm), and the mass is almost equal to the mass of an atom. Electrons move around the nucleus, like planets around the Sun; the number of electrons in an uncharged (neutral) atom is such that their total negative charge compensates (neutralizes) the positive charge of the nucleus. Electrons must move around the nucleus, otherwise they would fall onto it under the influence of gravity. The difference between an atom and a planetary system is that in the latter there are gravitational forces, and in an atom there are electric (Coulomb) forces. Near the nucleus, which can be considered as a point positive charge, there is a very strong electric field. Therefore, flying near the nucleus, positively charged α-particles (helium nuclei) experience strong deflection (see. rice. 4
). Later it was found out (G. Moseley) that the charge of the nucleus increases from one chemical element to another by elementary unit charge, equal to the charge electron (but with positive sign). Numerically, the charge of the nucleus of an atom, expressed in units of elementary charge e, is equal to serial number corresponding element in the periodic table. To test the planetary model, Rutherford and his collaborator Charles Darwin calculated the angular distribution of α particles scattered by a point nucleus - the center of Coulomb forces. The obtained result was verified experimentally - by measuring the number of α-particles scattered at different angles. The results of the experiment coincided exactly with theoretical calculations, thereby brilliantly confirming Rutherford’s planetary model of the atom. However, the planetary model of the atom encountered fundamental difficulties. According to classical electrodynamics, a charged particle moving with acceleration continuously emits electromagnetic energy. Therefore, electrons, moving around the nucleus, i.e., accelerated, would have to continuously lose energy through radiation. But at the same time, in an insignificant fraction of a second they would lose all their kinetic energy and fall onto the core. Another difficulty, also associated with radiation, was the following: if we assume (in accordance with classical electrodynamics) that the frequency of the light emitted by an electron is equal to the frequency of oscillations of the electron in the atom (i.e., the number of revolutions it makes in its orbit in one second) or has a multiple of it, then the emitted light, as the electron approaches the nucleus, should continuously change its frequency, and the spectrum of the light emitted by it should be continuous. But this contradicts experience. The atom emits light waves well-defined frequencies typical for a given chemical element, and is characterized by a spectrum consisting of individual spectral lines - a line spectrum. A number of regularities were experimentally established in the line spectra of elements, the first of which was discovered by the Swiss scientist I. Balmer (1885) in the spectrum of hydrogen. The most general pattern - the combination principle - was found by the Austrian scientist W. Ritz (1908). This principle can be formulated as follows: for the atoms of each element, a sequence of numbers can be found T 1 ,T 2 ,T 3,... - so-called spectral terms such that the frequency v each spectral line of a given element is expressed as the difference of two terms: v = T k - T i .
For the hydrogen atom the term Tn = R/n 2, Where n- integer taking value n= 1, 2, 3,..., a R- so-called Rydberg constant (see Rydberg constant).
Thus, within the framework of Rutherford's model of the atom, the stability of the atom with respect to radiation and the line spectra of its radiation could not be explained. On its basis, neither the laws of thermal radiation nor the laws of photoelectric phenomena that arise when radiation interacts with matter could be explained. It turned out to be possible to explain these laws based on completely new quantum concepts, first introduced by the German physicist M. Planck (1900). To derive the law of energy distribution in the spectrum of thermal radiation - radiation from heated bodies - Planck suggested that atoms of matter emit electromagnetic energy (light) in the form of separate portions - light quanta, the energy of which is proportional to v(radiation frequency): E = hv, Where h- a constant characteristic of quantum theory and called Planck's constant (See Planck's constant). In 1905, A. Einstein gave a quantum explanation of photoelectric phenomena, according to which the energy of a quantum hv goes to tearing an electron out of a metal - Work function R - and to communicate kinetic energy to him T kin; hv = R+ Tkin. At the same time, Einstein introduced the concept of light quanta as a special kind of particles; these particles were subsequently called Photons.
It turned out that the contradictions of Rutherford's model could be resolved only by abandoning a number of conventional concepts of classical physics. The most important step in building the theory of the atom was made by the Danish physicist N. Bohr (1913). Bohr's postulates and Bohr's atomic model. Bohr laid the basis for the quantum theory of the atom on two postulates that characterize those properties of the atom that did not fit into the framework of classical physics. These Bohr postulates can be formulated as follows: 1. Existence of stationary states. The atom does not radiate and is stable only in some stationary (time-invariant) states corresponding to a discrete (discontinuous) series of “allowed” energy values E 1 ,E 2 ,E 3 ,
E 4,... Any change in energy is associated with a quantum (jump) transition from one stationary state to another. 2. Condition for radiation frequencies (quantum transitions with radiation). When transitioning from one stationary state with energy E i into another with energy E k an atom emits or absorbs light of a certain frequency v in the form of a radiation quantum (photon) hv, according to the ratio hv = E i - E k. When emitted, an atom moves from a state with higher energy E i to a state with lower energy E k , when absorbed, on the contrary, from a state with lower energy E k to a state with higher energy E i.
Bohr's postulates immediately allow us to understand physical meaning Ritz combination principle (see above); comparison of ratios hv = E i - E k and v = T k - T i
shows that the spectral terms correspond to stationary states, and the energy of the latter should be equal (up to a constant term) E i = -hT i ,E k = -hT k.
When light is emitted or absorbed, the energy of the atom changes; this change is equal to the energy of the emitted or absorbed photon, i.e. the law of conservation of energy takes place. The line spectrum of an atom is the result of discreteness possible values his energy. To determine the permissible values of the energy of an atom - quantization of its energy - and to find the characteristics of the corresponding stationary states, Bohr used classical (Newtonian) mechanics. “If we generally wish to form a visual representation of stationary states, we have no other means, at least for now, except ordinary mechanics,” Bohr wrote in 1913 (“Three articles on the spectra and structure of atoms,” M.-L., 1923, p. 22). For the simplest atom - a hydrogen atom, consisting of a nucleus with a charge + e(proton) and electron with charge - e, Bohr considered the motion of an electron around the nucleus in circular orbits. Comparing the energy of an atom E with spectral terms Tn = R/n 2 for the hydrogen atom, found with great accuracy from the frequencies of its spectral lines, he obtained the possible values of the energy of the atom E n= -hT n = -hR/n 2(where n= 1, 2, 3,...). They correspond to circular orbits of radius a n = a 0 n 2 , Where a 0 = 0.53·10 -8 cm - Bohr radius - the radius of the smallest circular orbit (at n= 1). Bohr calculated the circulation frequencies v electron around the nucleus in circular orbits depending on the energy of the electron. It turned out that the frequencies of light emitted by an atom do not coincide with the revolution frequencies v n as required classical electrodynamics, and are proportional, according to the relation hv = E i - E k, the difference in electron energies in two possible orbits. To find the connection between the electron's orbital frequency and the radiation frequency, Bohr made the assumption that the results of quantum and classical theories should coincide at low radiation frequencies (for long wavelengths; such a coincidence occurs for thermal radiation, the laws of which were derived by Planck). He equated for large n transition frequency v = (E n+1 - E n)/ h frequency of circulation v n in orbit with data n and calculated the value Rydberg constant R, which coincided with great accuracy with the value R, found from experience, which confirmed Bohr's assumption. Bohr also managed not only to explain the spectrum of hydrogen, but also to convincingly show that some spectral lines that were attributed to hydrogen belong to helium. Bohr's assumption that the results of quantum and classical theories should coincide in the limiting case of low radiation frequencies represented the original form of the so-called. principle of correspondence. Subsequently, Bohr successfully used it to find the intensities of spectral lines. As the development of modern physics has shown, the correspondence principle turned out to be very general (see Correspondence principle) .
In Bohr's atomic theory, the quantization of energy, i.e., finding its possible values, turned out to be a special case general method finding “allowed” orbits. According to quantum theory, such orbits are only those for which the angular momentum of the electron in the atom is equal to an integer multiple h/2π. Each allowed orbit corresponds to a certain possible value of the energy of the atom (see Atom).
The main provisions of the quantum theory of the atom - Bohr's 2 postulates - were comprehensively confirmed experimentally. Particularly clear confirmation was provided by the experiments of the German physicists J. Frank and G. Hertz (1913-16). The essence of these experiments is this. A stream of electrons, the energy of which can be controlled, enters a vessel containing mercury vapor. The electrons are given energy, which gradually increases. As the electron energy increases, the current in the galvanometer connected to electrical circuit, increases; when the electron energy turns out to be equal to certain values (4.9; 6.7; 10.4 ev), the current drops sharply ( rice. 5
). At the same time, it can be found that mercury vapor is emitted ultraviolet rays a certain frequency. The facts presented allow only one interpretation. While the electron energy is less than 4.9 ev, electrons do not lose energy when colliding with mercury atoms - the collisions are elastic in nature. When the energy turns out to be equal to a certain value, exactly 4.9 ev, electrons transfer their energy to mercury atoms, which then emit it in the form of quanta of ultraviolet light. The calculation shows that the energy of these photons is equal to exactly the energy that the electrons lose. These experiments proved that the internal energy of an atom can only have certain discrete values, that the atom absorbs energy from the outside and emits it immediately in whole quanta and that, finally, the frequency of the light emitted by the atom corresponds to the energy lost by the atom. Further development of A. f. showed the validity of Bohr's postulates not only for atoms, but also for other microscopic systems - for molecules and for atomic nuclei. These postulates should be considered as firmly established experimental quantum laws. They constitute that part of Bohr's theory that was not only preserved during the further development of quantum theory, but also received its justification. The situation is different with Bohr's model of the atom, which is based on considering the movement of electrons in an atom according to the laws of classical mechanics with the imposition of additional quantization conditions. This approach made it possible to obtain a number of important results, but was inconsistent: quantum postulates were artificially attached to the laws of classical mechanics. A consistent theory was created in the 20s. 20th century Quantum mechanics. Its creation was prepared by the further development of model concepts of Bohr's theory, during which its strengths and weaknesses became clear. Development of Bohr's model theory of the atom. Very important result Bohr's theory was an explanation of the spectrum of the hydrogen atom. A further step in the development of the theory of atomic spectra was made by the German physicist A. Sommerfeld. Having developed in more detail the rules of quantization, based on a more complex picture of the movement of electrons in an atom (along elliptical orbits) and taking into account the screening of the external (so-called valence) electron in the field of the nucleus and internal electrons, he was able to explain a number of patterns in the spectra of alkali metals. Bohr's atomic theory also shed light on the structure of the so-called. characteristic spectra of X-ray radiation. The X-ray spectra of atoms, just like their optical spectra, have a discrete line structure characteristic of a given element (hence the name). While studying the characteristic X-ray spectra of various elements, the English physicist G. Moseley discovered the following pattern: the square roots of the frequencies of emitted lines increase uniformly from element to element throughout Mendeleev’s periodic system in proportion to the atomic number of the element. It is interesting that Moseley's law completely confirmed the correctness of Mendeleev, who in some cases violated the principle of placing elements in the table according to increasing atomic weight and placed some heavier elements ahead of lighter ones. Based on Bohr's theory, it was possible to explain the periodicity of the properties of atoms. In a complex atom, electron shells are formed, which are sequentially filled, starting from the innermost, with certain numbers of electrons (the physical reason for the formation of shells became clear only on the basis of the Pauli principle, see below). The structure of the outer electron shells repeats periodically, which determines the periodic repeatability of the chemical and many physical properties of elements located in the same group periodic table. On the basis of Bohr's theory, the German chemist W. Kossel explained (1916) chemical interactions in the so-called. heteropolar molecules. However, not all questions of atomic theory could be explained on the basis of the model concepts of Bohr's theory. It did not cope with many problems of the theory of spectra; it allowed one to obtain only the correct values of the frequencies of the spectral lines of the hydrogen atom and hydrogen-like atoms, while the intensities of these lines remained unexplained; Bohr had to apply the correspondence principle to explain the intensities. When moving to explain the movements of electrons in atoms more complex than the hydrogen atom, Bohr's model theory found itself at a dead end. Already the helium atom, in which 2 electrons move around the nucleus, did not give in theoretical interpretation based on it. The difficulties were not limited to quantitative discrepancies with experience. The theory also turned out to be powerless in solving such a problem as combining atoms into a molecule. Why do 2 neutral hydrogen atoms combine to form a hydrogen molecule? How can we explain the nature of valence? What connects atoms solid? These questions remained unanswered. Within the framework of Bohr's model it was impossible to find an approach to solving them. Quantum mechanical theory of the atom. The limitations of the Bohr model of the atom were rooted in the limitations of classical ideas about the movement of microparticles. It became clear that for the further development of atomic theory it is necessary to critically reconsider the basic concepts of the movement and interaction of microparticles. The unsatisfactory nature of the model based on classical mechanics with the addition of quantization conditions was clearly understood by Bohr himself, whose views had a great influence on further development A.f. The beginning of a new stage in the development of A. f. was inspired by the idea expressed by the French physicist L. de Broglie (1924) about the dual nature of the movement of micro-objects, in particular the electron (see De Broglie Waves). This idea became the starting point of quantum mechanics (See Quantum mechanics), created in 1925-26 by the works of W. Heisenberg and M. Born (Germany), E. Schrödinger (Austria) and P. Dirac (England), and developed on its basis modern quantum mechanical theory of the atom. The concepts of quantum mechanics about the movement of an electron (in general, microparticles) are fundamentally different from classical ones. According to quantum mechanics, an electron does not move along a trajectory (orbit) like a solid ball; The motion of the electron also has some features characteristic of wave propagation. On the one hand, an electron always acts (for example, in collisions) as a single whole, as a particle with an indivisible charge and mass; at the same time, electrons with a certain energy and momentum propagate like a plane wave with a certain frequency (and a certain wavelength). Electron energy E How are particles related to frequency? v electron wave ratio: E=hv, and its impulse r - with wavelength λ
ratio: р = h/λ.
The stable motions of an electron in an atom, as shown by Schrödinger (1926), are in some respects similar to standing waves (See standing waves) ,
the amplitudes of which are different at different points. Moreover, in an atom, as in oscillatory system, only some “selected” movements are possible with certain values of energy, angular momentum and projection of the angular momentum of the electron in the atom. Each stationary state of an atom is described using some wave function(See wave function) ,
which is the solution wave equation a special type - the Schrödinger equation; The wave function corresponds to an “electron cloud”, which characterizes (on average) the distribution of the electron charge density in the atom (see Atom ,
right there on rice. 3
projections of the “electron clouds” of the hydrogen atom are shown). In the 20-30s. Approximate methods for calculating the distribution of electron charge density in complex atoms were developed, in particular the Thomas-Fermi method (1926, 1928). This value and the associated value of the so-called. atomic factor (See Atomic factor)
important in the study of electron collisions with atoms, as well as scattering by them x-rays. Based on quantum mechanics, it was possible to correctly calculate the energies of electrons in complex atoms by solving the Schrödinger equation. Approximate methods for such calculations were developed in 1928 by D. Hartree (England) and in 1930 by V. A. Fock (USSR). Studies of atomic spectra completely confirmed the quantum mechanical theory of the atom. It turned out that the state of an electron in an atom significantly depends on its Spin a -
own mechanical moment of momentum. An explanation was given for the action of external electric and magnetic fields on the atom (see Stark phenomenon (See Stark effect), Zeeman phenomenon). Important general principle, associated with the electron spin, was discovered by the Swiss physicist W. Pauli (1925) (see Pauli principle), according to this principle, only one electron can be in each electronic state in an atom; If this state is already occupied by some electron, then the subsequent electron, entering the composition of the atom, is forced to occupy another state. Based on the Pauli principle, the filling numbers of electron shells in complex atoms, which determine the periodicity of the properties of elements, were finally established. Based on quantum mechanics, German physicists W. Heitler and F. London (1927) gave the theory of the so-called. homeopolar chemical bond of two identical atoms (for example, hydrogen atoms in the H 2 molecule), which cannot be explained within the framework of the Bohr model of the atom. Important applications of quantum mechanics in the 30s. and later there were studies bonded atoms, included in the molecule or crystal. The states of an atom that is part of a molecule differ significantly from the states of a free atom. The atom also undergoes significant changes in a crystal under the influence of an intracrystalline field, the theory of which was first developed by H. Bethe (1929). By studying these changes, it is possible to establish the nature of the interaction of the atom with its environment. The largest experimental achievement in this area of A. f. was the discovery by E.K. Zavoisky in 1944 of electron paramagnetic resonance (See Electron paramagnetic resonance) ,
which made it possible to study the various connections of atoms with the environment. Modern atomic physics. The main sections of modern A. f. are atomic theory, atomic (optical) spectroscopy, X-ray spectroscopy, radio spectroscopy (it also studies rotational levels of molecules), physics of atomic and ionic collisions. Various branches of spectroscopy cover different ranges radiation frequencies and, accordingly, different ranges of quantum energies. While X-ray spectroscopy studies the radiation of atoms with quantum energies of up to hundreds of thousands. ev, radio spectroscopy deals with very small quanta - down to quanta less than 10 -6 ev.
The most important task of A. f. - detailed determination of all characteristics of atomic states. We are talking about determining the possible values of the energy of an atom - its energy levels, values of angular momentum and other quantities characterizing the states of the atom. Fine and hyperfine structures of energy levels are studied (see Atomic spectra) ,
changes in energy levels under the influence of electric and magnetic fields - both external, macroscopic, and internal, microscopic. Of great importance is such a characteristic of atomic states as the lifetime of an electron at the energy level. Finally, much attention is paid to the mechanism of excitation of atomic spectra. The areas of phenomena studied by different sections of physics overlap. X-ray spectroscopy by measuring the emission and absorption of x-rays makes it possible to determine mainly the binding energies of internal electrons with the nucleus of an atom (ionization energy) and the distribution of the electric field inside the atom. Optical spectroscopy studies sets of spectral lines emitted by atoms, determines the characteristics of atomic energy levels, the intensities of spectral lines and the associated lifetimes of the atom in excited states, the fine structure of energy levels, their displacement and splitting in electric and magnetic fields. Radio spectroscopy studies in detail the width and shape of spectral lines, their hyperfine structure, shift and splitting in a magnetic field, and in general intra-atomic processes caused by very weak interactions and environmental influences. Analysis of the results of collisions of fast electrons and ions with atoms makes it possible to obtain information about the distribution of electron charge density (“electron cloud”) inside the atom, about the excitation energies of the atom, and ionization energies. The results of a detailed study of the structure of atoms find the widest applications not only in many branches of physics, but also in chemistry, astrophysics and other fields of science. Based on the study of the broadening and shift of spectral lines, one can judge the local fields in the medium (liquid, crystal) that cause these changes, and the state of this medium (temperature, density, etc.). Knowledge of the distribution of electron charge density in an atom and its changes during external interactions makes it possible to predict the type chemical bonds, which an atom can form, the behavior of an ion in a crystal lattice. Information about the structure and characteristics of the energy levels of atoms and ions is extremely important for quantum electronics devices (See.