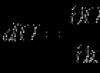Another class of clusters were elongated cylindrical carbon formations, which later, after their structure was elucidated, were called " carbon nanotubes"(CNTs). CNTs are large, sometimes even ultra-large (over 10 6 atoms) molecules built from carbon atoms.
Typical structural scheme single-walled CNT and the result of computer calculation of it molecular orbitals shown in Fig. 3.1. At the vertices of all hexagons and pentagons, shown as white lines, there are carbon atoms in a state of sp 2 hybridization. To ensure that the structure of the CNT framework is clearly visible, the carbon atoms are not shown here. But they are not difficult to imagine. The gray tone shows the appearance of the molecular orbitals of the lateral surface of the CNT.
Fig 3.1
The theory shows that the structure of the side surface of a single-walled CNT can be imagined as one layer of graphite rolled into a tube. It is clear that this layer can be folded only in those directions in which the hexagonal lattice is aligned with itself when closed cylindrical surface. Therefore, CNTs have only a certain set of diameters and are classified By vectors indicating the direction of folding of the hexagonal lattice. Both the appearance and variations in the properties of CNTs depend on this. Three typical options are shown in Fig. 3.2.
The set of possible CNT diameters overlaps range from slightly less than 1 nm to many tens of nanometers. A length CNTs can reach tens of micrometers. Record By The length of CNTs has already exceeded the limit of 1 mm.
Sufficiently long CNTs (when length much larger in diameter) can be considered as a one-dimensional crystal. On them one can distinguish a “unit cell”, which is repeated many times along the axis of the tube. And this is reflected in some of the properties of long carbon nanotubes.
Depending on the rollup vector of the graphite layer (experts say: “from chirality") nanotubes can be both conductors and semiconductors. CNTs of the so-called “saddle” structure always have a fairly high, “metallic” electrical conductivity.

Rice. 3.2
The “lids” that close the CNTs at the ends may also be different. They have the shape of “halves” of different fullerenes. Their main options are shown in Fig. 3.3.

Rice. 3.3 The main options for “covers” of single-walled CNTs
There are also multiwalled CNTs. Some of them look like a layer of graphite rolled into a scroll. But most consist of single-layer tubes inserted into one another, interconnected by van der Waals forces. If single-walled CNTs are almost always covered with lids, then multiwalled CNTs They are also partially open. They usually exhibit many more small structural defects than single-walled CNTs. Therefore, for applications in electronics, preference is still given to the latter.
CNTs grow not only straight, but also curvilinear, bent to form a “knee,” and even completely rolled up in the form of a torus. Often, several CNTs are tightly connected to each other and form “bundles”.
Materials used for nanotubes
The development of methods for the synthesis of carbon nanotubes (CNTs) has followed the path of lowering synthesis temperatures. After the creation of the technology for producing fullerenes, it was discovered that during electric arc evaporation of graphite electrodes, along with the formation of fullerenes, extended cylindrical structures are formed. Microscopist Sumio Iijima, using a transmission electron microscope (TEM), was the first to identify these structures as nanotubes. High-temperature methods for producing CNTs include the electric arc method. If you evaporate a graphite rod (anode) in an electric arc, then a hard carbon build-up (deposit) is formed on the opposite electrode (cathode), the soft core of which contains multi-walled CNTs with a diameter of 15-20 nm and a length of more than 1 μm.
The formation of CNTs from fullerene soot under high-temperature thermal influence on soot was first observed by Oxford and Swiss groups. The installation for electric arc synthesis is metal-intensive and energy-consuming, but is universal for producing various types of carbon nanomaterials. A significant problem is the non-equilibrium process during arc combustion. The electric arc method at one time replaced the method of laser evaporation (ablation) with a laser beam. The ablation unit is a conventional resistive heating oven producing a temperature of 1200°C. To obtain higher temperatures in it, it is enough to place a carbon target in the furnace and direct a laser beam at it, alternately scanning the entire surface of the target. Thus, Smalley’s group, using expensive installations with a short-pulse laser, obtained nanotubes in 1995, “significantly simplifying” the technology of their synthesis.
However, the yield of CNTs remained low. The introduction of small additions of nickel and cobalt (0.5 at.%) into graphite made it possible to increase the CNT yield to 70-90%. From this moment on, a new stage began in understanding the mechanism of nanotube formation. It became obvious that the metal was a catalyst for growth. This is how the first works appeared on the production of nanotubes by a low-temperature method - the method of catalytic pyrolysis of hydrocarbons (CVD), where iron group metal particles were used as a catalyst. One of the installation options for producing nanotubes and nanofibers CVD method is a reactor into which an inert carrier gas is supplied, carrying the catalyst and hydrocarbon to a high-temperature zone.
In a simplified way, the growth mechanism of CNTs is as follows. The carbon formed during the thermal decomposition of hydrocarbons dissolves in the metal nanoparticle. When a high concentration of carbon in a particle is reached, an energetically favorable “release” of excess carbon occurs on one of the faces of the catalyst particle in the form of a distorted semifulerene cap. This is how a nanotube is born. The decomposed carbon continues to enter the catalyst particle, and in order to dump its excess concentration in the melt, it is necessary to constantly get rid of it. The rising hemisphere (semi-fullerene) from the surface of the melt carries with it dissolved excess carbon, the atoms of which form outside the melt S-S connection, which is a cylindrical nanotube frame.
The melting temperature of a particle in a nanosized state depends on its radius. How smaller radius, the lower the melting point, due to the Gibbs-Thompson effect. Therefore, iron nanoparticles with a size of about 10 nm are in a molten state below 600°C. On this moment Low-temperature synthesis of CNTs was carried out using the catalytic pyrolysis of acetylene in the presence of Fe particles at 550°C. Reducing the synthesis temperature also has negative consequences. At lower temperatures, CNTs with a large diameter (about 100 nm) and a highly defective structure such as “bamboo” or “nested nanocones” are obtained. The resulting materials consist only of carbon, but they do not even come close to the extraordinary characteristics (for example, Young's modulus) observed in single-walled carbon nanotubes obtained by laser ablation or electric arc synthesis.
Structure and classification of nanotubes
Carbon nanotubes(carbon nanotubes, CNTs) are molecular compounds belonging to the class of allotropic modifications of carbon. They are extended cylindrical structures with a diameter from one to several tens of nanometers and a length from one to several microns.
Figure 8. Carbon nanotube
Nanotubes consist of one or more layers rolled into a tube, each of which represents a hexagonal network of graphite (graphene), the basis of which is hexagons with carbon atoms located at the vertices of the corners. In all cases, the distance between layers is 0.34 nm, that is, the same as between layers in crystalline graphite.
The upper ends of the tubes are closed with hemispherical caps, each layer of which is composed of hexagons and pentagons, reminiscent of the structure of half a fullerene molecule.
It is believed that the discoverer of carbon nanotubes is an employee of the Japanese NEC corporation, Sumio Iijima, who in 1991 observed the structures of multi-walled nanotubes while studying under an electron microscope the sediments that formed during the synthesis of molecular forms of pure carbon with a cellular structure.
An ideal nanotube is a graphite plane rolled into a cylinder, i.e. a surface lined with regular hexagons, at the vertices of which carbon atoms are located.
The parameter indicating the coordinates of the hexagon, which, as a result of folding the plane, should coincide with the hexagon located at the origin of coordinates, is called the chirality of the nanotube. The chirality of a nanotube determines its electrical characteristics.
As observations made using electron microscopes have shown, most nanotubes consist of several graphite layers, either nested one inside the other or wound on a common axis.
Single-walled nanotubes(single-walled nanotubes, SWNTs) are the simplest type of nanotubes. Most have a diameter of about 1 nm with a length that can be many thousands of times greater.

Figure 9. Model of a single-walled nanotube.
Such a tube ends with hemispherical vertices containing, along with regular hexagons, also six regular pentagons.
The structure of single-walled nanotubes observed experimentally differs in many respects from the idealized picture presented above. First of all, this concerns the vertices of the nanotube, the shape of which, as follows from observations, is far from an ideal hemisphere.

Figure 10. Models cross section multiwalled nanotubes
Multiwalled nanotubes differ from single-walled nanotubes in a much wider variety of shapes and configurations, both in the longitudinal and transverse directions. Possible types of transverse structure of multiwalled nanotubes are presented in Figure 10.
The “Russian dolls” type structure is a collection of single-walled nanotubes coaxially nested into each other ( Figure 10 a). The last of the structures shown (Figure 10 b) resembles a scroll. For the above structures, the distances between adjacent graphite layers are close to 0.34 nm, i.e. the distance between adjacent planes of crystalline graphite. The implementation of a particular structure in a specific experimental situation depends on the conditions of nanotube synthesis. 2.2 Preparation of carbon nanotubes
The most common methods for synthesizing nanotubes are electric arc method, laser ablation and chemical vapor deposition (CVD).
Arc discharge - The essence of this method is to obtain carbon nanotubes in plasma arc discharge, burning in a helium atmosphere, in technological installations for the production of fullerenes. However, other arc burning modes are used here: low densities arc current, higher helium pressure (~500 Torr), larger diameter cathodes. To obtain the maximum number of nanotubes, the arc current must be 65-75 A, voltage - 20-22 V, electron plasma temperature - about 4000 K. Under these conditions, the graphite anode intensively evaporates, delivering individual atoms or pairs of carbon atoms, of which the cathode or on the water-cooled walls of the chamber and carbon nanotubes are formed.
To increase the yield of nanotubes in the sputtering products, a catalyst (a mixture of iron group metals) is introduced into the graphite rod and the pressure is changed inert gas and spray mode.
The content of nanotubes in the cathode deposit reaches 60%. The resulting nanotubes, up to 40 microns in length, grow from the cathode perpendicular to its surface and are combined into cylindrical bundles with a diameter of about 50 nm.
A typical diagram of an electric arc installation for the production of material containing nanotubes and fullerenes, as well as other carbon formations, is shown in Figure 11.

Figure 11. Scheme of the installation for producing nanotubes using the electric arc method.
The laser ablation method was invented by Richard Smalley and employees at Rice University and is based on the evaporation of a graphite target in a high-temperature reactor. Nanotubes appear on the cooled surface of the reactor as graphite evaporation condensate. A water-cooled surface can be included in a nanotube collection system. The product yield in this method is about 70%. It is used to produce predominantly single-walled carbon nanotubes with a diameter controlled by the reaction temperature. However, the cost of this method is much more expensive than others.
Chemical vapor deposition (CVD) - a method of catalytic deposition of carbon vapor was discovered back in 1959, but until 1993 no one imagined that nanotubes could be obtained in this process.

Figure 12. Diagram of the installation for producing nanotubes by chemical deposition.
Fine metal powder (most often nickel, cobalt, iron or combinations thereof) is used as a catalyst, which is poured into a ceramic crucible located in a quartz tube. The latter, in turn, is placed in a heating device that allows maintaining a controlled temperature in the range from 700 to 1000°C. A mixture of hydrocarbon gas and buffer gas is purged through a quartz tube. Typical composition of a mixture of C 2 H 2: N 2 in a ratio of 1:10. The process can last from several minutes to several hours. Long carbon filaments and multiwalled nanotubes up to several tens of micrometers in length with an internal diameter of 10 nm and an external diameter of 100 nm grow on the surface of the catalyst. The diameter of nanotubes grown in this way depends on the size of the metal particles.
This mechanism is the most common commercial method for producing carbon nanotubes. Among other methods for producing nanotubes, CVD is the most promising on an industrial scale due to the best ratio in terms of unit price. In addition, it allows the preparation of vertically oriented nanotubes on the desired substrate without additional collection, as well as controlling their growth through a catalyst.
Broad prospects for the use of nanotubes in materials science open up when superconducting crystals (for example, TaC) are encapsulated inside carbon nanotubes. The possibility of obtaining superconducting crystals encapsulated in nanotubes makes it possible to isolate them from harmful effects external environment, for example, from oxidation, thereby opening the way to more effective development relevant nanotechnologies.
The large negative magnetic susceptibility of nanotubes indicates their diamagnetic properties. It is assumed that the diamagnetism of nanotubes is due to the flow of electron currents around their circumference. The magnitude of the magnetic susceptibility does not depend on the orientation of the sample, which is associated with its disordered structure.
The basis for many technological applications of nanotubes is their property of high specific surface area (in the case of a single-walled nanotube, about 600 sq. m per 1/g), which opens up the possibility of their use as a porous material in filters, etc.
Nanotube material can be successfully used as a supporting substrate for implementing heterogeneous catalysis, and catalytic activity open nanotubes noticeably exceeds the corresponding parameter for closed nanotubes.
It is possible to use nanotubes with a high specific surface area as electrodes for electrolytic capacitors with high specific power. Carbon nanotubes have proven themselves well in experiments using them as a coating that promotes the formation of a diamond film.
Such properties of a nanotube as its small size, which varies significantly depending on the synthesis conditions, electrical conductivity, mechanical strength and chemical stability, allow us to consider the nanotube as the basis for future microelectronic elements.
Nanotubes can serve as the basis for extremely thin measuring instruments used to monitor surface irregularities in electronic circuits.
Interesting applications can be obtained by nanotubes when filled with various materials. In this case, the nanotube can be used both as a carrier of the material filling it, and as an insulating shell that protects this material from electrical contact or from chemical interaction with surrounding objects.
Ministry of Education and Science of the Russian Federation
Federal government agency higher vocational education
Russian Chemical-Technological University named after. D. I. Mendeleeva
Faculty of Petroleum Chemistry and Polymer Materials
Department chemical technology carbon materials
PRACTICE REPORT
on the topic CARBON NANOTUBES AND NANOVOLVES
Completed by: Marinin S. D.
Checked by: Doctor of Chemical Sciences, Bukharkina T.V.
Moscow, 2013
Introduction
The field of nanotechnology is considered worldwide to be a key topic for 21st century technology. The possibilities of their versatile application in such areas of the economy as semiconductor production, medicine, sensor technology, ecology, automotive, building materials, biotechnology, chemistry, aviation and astronautics, mechanical engineering and the textile industry, carry enormous growth potential. The use of nanotechnology products will save on raw materials and energy consumption, reduce emissions into the atmosphere, and thereby contribute to sustainable economic development.
Developments in the field of nanotechnology are being carried out by a new interdisciplinary field - nanoscience, one of the areas of which is nanochemistry. Nanochemistry arose at the turn of the century, when it seemed that everything in chemistry was already open, everything was clear, and all that remained was to use the acquired knowledge for the benefit of society.
Chemists have always known and well understood the importance of atoms and molecules as the main “building blocks” of a huge chemical foundation. At the same time, the development of new research methods, such as electron microscopy, highly selective mass spectroscopy, in combination with special methods of sample preparation, has made it possible to obtain information about particles containing a small number of atoms, less than a hundred.
Such particles measuring about 1 nm (10-9 m is just a millimeter divided by a million) have unusual, difficult to predict Chemical properties.
The most well-known and understandable to most people are the following nanostructures: fullerenes, graphene, carbon nanotubes and nanofibers. They all consist of carbon atoms bonded to each other, but their shape differs significantly. Graphene is a plane, a monolayer, a "blanket" of carbon atoms in SP 2 hybridization. Fullerenes are closed polygons, somewhat reminiscent of a soccer ball. Nanotubes - cylindrical hollow volumetric bodies. Nanofibers can be in the form of cones, cylinders, or bowls. In my work I will try to highlight nanotubes and nanofibers.
Structure of nanotubes and nanofibers
What are carbon nanotubes? Carbon nanotubes are a carbon material that are cylindrical structures with a diameter of the order of several nanometers, consisting of graphite planes rolled into a tube. The graphite plane is a continuous hexagonal network with carbon atoms at the vertices of the hexagons. Carbon nanotubes can vary in length, diameter, chirality (the symmetry of the folded graphite plane), and the number of layers. Chirality<#"280" src="doc_zip1.jpg" />
Single-walled nanotubes. Single-walled carbon nanotubes (SWCNTs) are a subtype of carbon nanofibers with a structure formed by rolling graphene into a cylinder and connecting its sides without a seam. Rolling graphene into a cylinder without a seam is possible only in a finite number of ways, differing in the direction of the two-dimensional vector that connects two equivalent points on the graphene that coincide when it is rolled into a cylinder. This vector is called the chirality vector single-walled carbon nanotube. Thus, single-walled carbon nanotubes differ in diameter and chirality. The diameter of single-walled nanotubes, according to experimental data, varies from ~ 0.7 nm to ~ 3-4 nm. The length of a single-walled nanotube can reach 4 cm. There are three forms of SWCNTs: achiral “chair” type (two sides of each hexagon are oriented perpendicular to the CNT axis), achiral “zigzag” type (two sides of each hexagon are oriented parallel to the CNT axis) and chiral or helical (each the side of the hexagon is located to the CNT axis at an angle different from 0 and 90 º ). Thus, achiral CNTs of the “chair” type are characterized by indices (n,n), of the “zigzag” type - (n,0), chiral - (n,m).
Multi-walled nanotubes. Multiwalled carbon nanotubes (MWCNTs) are a subtype of carbon nanofibers with a structure formed by several single-walled carbon nanotubes nested inside each other (see Fig. 2). The outer diameter of multiwalled nanotubes varies widely from a few nanometers to tens of nanometers.
The number of layers in MWCNTs is most often no more than 10, but in some cases reaches several tens.
Sometimes, among multiwalled nanotubes, double-walled nanotubes are distinguished as a special type. The “Russian dolls” type structure is a collection of cylindrical tubes coaxially nested into each other. Another variation of this structure is a collection of coaxial prisms nested within each other. Finally, the last of the above structures resembles a scroll. For all structures in Fig. characteristic value of the distance between adjacent graphene layers is close to the value of 0.34 nm, inherent in the distance between adjacent planes of crystalline graphite<#"128" src="doc_zip3.jpg" />
Russian Matryoshka Roll of Papier-mâché
Carbon nanofibers (CNFs) are a class of materials in which curved graphene layers, or nanocones, are folded into a one-dimensional thread whose internal structure can be characterized by an angle? between the graphene layers and the fiber axis. One common difference is between two main types of fibers: Herringbone, with densely packed conical graphene layers and large ones, and Bamboo, with cylindrical cup-like graphene layers and small ones, which are more like multi-walled carbon nanotubes.<#"228" src="doc_zip4.jpg" />
a - “coin column” nanofiber;
b - nanofiber “herringbone structure” (stack of cones, “fish bone”);
c - nanofiber “stack of cups” (“lamp shades”);
d - nanotube "Russian nesting doll";
d - bamboo-shaped nanofiber;
e - nanofiber with spherical sections;
g - nanofiber with polyhedral sections
The identification of carbon nanotubes as a separate subspecies is due to the fact that their properties differ markedly in better side from the properties of other types of carbon nanofibers. This is explained by the fact that the graphene layer, which forms the wall of the nanotube along its entire length, has high tensile strength, thermal and electrical conductivity. In contrast, in carbon nanofibers, when moving along the wall, transitions from one graphene layer to another occur. The presence of interlayer contacts and high defectiveness of the structure of nanofibers significantly worsens their physical characteristics.
Story
It is difficult to talk about the history of nanotubes and nanofibers separately, because these products often accompany each other during synthesis. One of the first data on the production of carbon nanofibers is probably the patent from 1889 for the production of tubular forms of carbon formed by the pyrolysis of a mixture of CH4 and H2 in an iron crucible by Hughes and Chambers. They used a mixture of methane and hydrogen to grow carbon filaments by pyrolyzing the gas followed by carbon deposition. It became possible to talk about obtaining these fibers for sure much later, when it became possible to study their structure using an electron microscope. The first observation of carbon nanofibers using electron microscopy was made in the early 1950s by Soviet scientists Radushkevich and Lukyanovich, who published a paper in the Soviet Journal of Physical Chemistry showing hollow graphitic carbon fibers that were 50 nanometers in diameter. In the early 1970s, Japanese researchers Koyama and Endo succeeded in producing vapor deposition carbon fibers (VGCF) with a diameter of 1 micron and a length of more than 1 mm. Later, in the early 1980s, Tibbetts in the USA and Benissad in France continued to improve the process for producing carbon fibers (VGCF). In the US, more in-depth research into the synthesis and properties of these materials for practical applications was carried out by R. Terry C. Baker and was motivated by the need to suppress the growth of carbon nanofibers due to persistent problems caused by material accumulation in various commercial processes, especially in the field of petroleum refining . The first attempt to commercialize carbon fibers grown from the gas phase was made by the Japanese company Nikosso in 1991 under the brand name Grasker, in the same year Ijima published his famous article, reporting the discovery of carbon nanotubes<#"justify">Receipt
Currently, syntheses based on the pyrolysis of hydrocarbons and the sublimation and desublimation of graphite are mainly used.
Sublimation-desublimation of graphitecan be implemented in several options:
- electric arc method,
- radiation heating (using solar concentrators or laser radiation),
- laser-thermal,
- heating by an electron or ion beam,
- sublimation in plasma,
- resistive heating.
Many of these options have their own variations. The hierarchy of some of the options for the electric arc method is shown in the diagram:
Currently, the most common method is thermal sputtering of graphite electrodes in arc discharge plasma. The synthesis process is carried out in a chamber filled with helium under a pressure of about 500 mm Hg. Art. When the plasma burns, intense thermal evaporation of the anode occurs, and a deposit forms on the end surface of the cathode, in which carbon nanotubes form. The maximum number of nanotubes is formed when the plasma current is minimal and its density is about 100 A/cm2. IN experimental facilities the voltage between the electrodes is about 15-25 V, the discharge current is several tens of amperes, the distance between the ends of the graphite electrodes is 1-2 mm. During the synthesis process, about 90% of the anode mass is deposited on the cathode. The resulting numerous nanotubes are about 40 µm long. They grow on the cathode perpendicularly flat surface its end and collected into cylindrical bundles with a diameter of about 50 microns.
Bundles of nanotubes regularly cover the surface of the cathode, forming a honeycomb structure. The content of nanotubes in the carbon deposit is about 60%. To separate the components, the resulting precipitate is placed in methanol and treated with ultrasound. The result is a suspension, which, after adding water, is separated in a centrifuge. Large particles stick to the walls of the centrifuge, and the nanotubes remain floating in suspension. Then the nanotubes are washed in nitric acid and dried in a gaseous flow of oxygen and hydrogen in a ratio of 1:4 at a temperature of 750 0C for 5 minutes. As a result of this processing, a lightweight porous material is obtained, consisting of numerous nanotubes with an average diameter of 20 nm and a length of 10 microns. So far, the maximum achieved nanofiber length is 1 cm.
Pyrolysis of hydrocarbons
In terms of the choice of starting reagents and methods of conducting processes, this group has a significantly larger number of options than the methods of sublimation and desublimation of graphite. It provides more precise control over the process of CNT formation, is more suitable for large-scale production and allows the production of not only carbon nanomaterials themselves, but also certain structures on substrates, macroscopic fibers consisting of nanotubes, as well as composite materials, in particular, modified with carbon CNTs carbon fibers and carbon paper, ceramic composites. Using recently developed nanosphere lithography, it was possible to obtain photonic crystals from CNTs. In this way, it is possible to isolate CNTs of a certain diameter and length.
The advantages of the pyrolytic method, in addition, include the possibility of its implementation for matrix synthesis, for example using porous alumina membranes or molecular sieves. Using aluminum oxide, it is possible to obtain branched CNTs and CNT membranes. The main disadvantages of the matrix method are the high cost of many matrices, their small size and the need to use active reagents and harsh conditions for dissolving the matrices.
Most often, for the synthesis of CNTs and CNFs, the processes of pyrolysis of three hydrocarbons: methane, acetylene and benzene, as well as the thermal decomposition (disproportionation) of CO are used. Methane, like carbon monoxide, is not prone to decomposition at low temperatures (non-catalytic decomposition of methane begins at ~900 O C), which makes it possible to synthesize SWCNTs with a relatively small amount of amorphous carbon impurity. Carbon monoxide does not decompose at low temperatures for another reason: kinetic. The difference in the behavior of various substances is visible in Fig. 94.
The advantages of methane over other hydrocarbons and carbon monoxide include the fact that its pyrolysis with the formation of CNTs or CNFs is combined with the release of H 2and can be used in existing H2 production facilities .
Catalysts
Catalysts for the formation of CNTs and CNFs are Fe, Co and Ni; promoters, which are introduced in smaller quantities, are predominantly Mo, W or Cr (less often - V, Mn, Pt and Pd), catalyst carriers are non-volatile oxides and hydroxides of metals (Mg, Ca, Al, La, Si, Ti, Zr) , solid solutions, some salts and minerals (carbonates, spinels, perovskites, hydrotalcite, natural clays, diatomites), molecular sieves (in particular, zeolites), silica gel, airgel, aluminum gel, porous Si and amorphous C. In this case, V, Cr, Mo, W, Mn and, probably, some other metals under pyrolysis conditions are in the form of compounds - oxides, carbides, metallates, etc.
Can be used as catalysts precious metals(Pd, Ru, PdSe), alloys (mischmetal, permalloy, nichrome, monel, stainless steel, Co-V, Fe-Cr, Fe-Sn, Fe-Ni-Cr, Fe-Ni-C, Co-Fe-Ni , hard alloy Co-WC, etc.), CoSi 2and CoGe 2,LaNi 5, MmNi 5(Mm - misch metal), alloys of Zr and other hydride-forming metals. On the contrary, Au and Ag inhibit the formation of CNTs.
Catalysts can be applied to silicon coated with a thin oxide film, to germanium, some types of glass, and substrates of other materials.
The ideal carrier for catalysts is porous silicon, obtained by electrochemical etching of single-crystalline silicon in a solution of a certain composition. Porous silicon may contain micropores (< 2 нм), мезопоры и макропоры (>100 nm). To obtain catalysts they use traditional methods:
- mixing (less commonly sintering) powders;
- sputtering or electrochemical deposition of metals onto a substrate with the subsequent transformation of a continuous thin film into nano-sized islands (layer-by-layer sputtering of several metals is also used;
- chemical vapor deposition;
- dipping the substrate into the solution;
- applying a suspension with catalyst particles to the substrate;
- applying the solution to a rotating substrate;
- impregnation of inert powders with salts;
- coprecipitation of oxides or hydroxides;
- ion exchange;
- colloidal methods (sol-gel process, reverse micelles method);
- thermal decomposition of salts;
- combustion of metal nitrates.
In addition to the two groups described above, a large number of other methods for producing CNTs have been developed. They can be classified according to the carbon sources used. The starting compounds are: graphite and other forms of solid carbon, organic compounds, inorganic compounds, organometallic compounds. Graphite can be converted into CNTs in several ways: intensive ball grinding followed by high-temperature annealing; electrolysis of molten salts; splitting into separate graphene sheets and subsequent spontaneous twisting of these sheets. Amorphous carbon can be converted into CNTs by treatment under hydrothermal conditions. CNTs were obtained from carbon black (soot) through high-temperature transformation in the presence of catalysts or without them, as well as through interaction with water vapor under pressure. Nanotubular structures are contained in vacuum annealing products (1000 O C) films of diamond-like carbon in the presence of a catalyst. Finally, the catalytic high-temperature transformation of fullerite C 60or its processing under hydrothermal conditions also leads to the formation of CNTs.
Carbon nanotubes exist in nature. A team of Mexican researchers discovered them in oil samples recovered from a depth of 5.6 km (Velasco-Santos, 2003). The diameter of CNTs ranged from several nanometers to tens of nanometers, and the length reached 2 μm. Some of them were filled with various nanoparticles.
Purification of carbon nanotubes
None of the common methods for obtaining CNTs makes it possible to isolate them in their pure form. Impurities in NT can be fullerenes, amorphous carbon, graphitized particles, and catalyst particles.
Three groups of CNT purification methods are used:
- destructive,
- non-destructive,
- combined.
Destructive methods use chemical reactions that can be oxidative or reductive and are based on differences in the reactivity of different carbon forms. For oxidation, either solutions of oxidizing agents or gaseous reagents are used, and hydrogen is used for reduction. Methods make it possible to isolate CNTs high purity, but are associated with tube losses.
Non-destructive methods include extraction, flocculation and selective precipitation, cross-flow microfiltration, size exclusion chromatography, electrophoresis, selective interaction with organic polymers. As a rule, these methods are low-productivity and ineffective.
Properties of carbon nanotubes
Mechanical. Nanotubes, as has been said, are an extremely strong material, both in tension and bending. Moreover, under the influence of mechanical stresses exceeding critical ones, nanotubes do not “break”, but are rearranged. Based on the high strength properties of nanotubes, it can be argued that they are the best material for a space elevator cable at the moment. As the results of experiments and numerical simulations show, the Young's modulus of a single-walled nanotube reaches values of the order of 1-5 TPa, which is an order of magnitude greater than that of steel. The graph below shows a comparison between a single-walled nanotube and high-strength steel.
According to calculations, the space elevator cable must withstand a mechanical stress of 62.5 GPa
Tensile diagram (dependence of mechanical stress ? from relative elongation?)
To demonstrate the significant difference between the current strongest materials and carbon nanotubes, let's conduct the following thought experiment. Let's imagine that, as previously assumed, a certain wedge-shaped structure will serve as a cable for the space elevator. homogeneous structure, consisting of the strongest materials available today, then the diameter of the cable at GEO (geostationary Earth orbit) will be about 2 km and will narrow to 1 mm at the surface of the Earth. In this case, the total mass will be 60 * 1010 tons. If carbon nanotubes were used as the material, then the diameter of the GEO cable would be 0.26 mm and 0.15 mm at the surface of the Earth, and therefore the total mass would be 9.2 tons. As can be seen from the above facts, carbon nanofiber is exactly the material that is needed when constructing a cable, the actual diameter of which will be about 0.75 m, in order to withstand also electromagnetic system, used to move the space elevator cabin.
Electrical. Due to the small size of carbon nanotubes, it was only in 1996 that it was possible to directly measure their specific electrical resistance four-pin method.
Gold stripes were applied to the polished surface of silicon oxide in a vacuum. Nanotubes 2–3 μm long were deposited into the gap between them. Then, 4 tungsten conductors with a thickness of 80 nm were applied to one of the nanotubes selected for measurement. Each of the tungsten conductors had contact with one of the gold strips. The distance between the contacts on the nanotube ranged from 0.3 to 1 μm. The results of direct measurements showed that the resistivity of nanotubes can vary within significant limits - from 5.1 * 10 -6up to 0.8 Ohm/cm. The minimum resistivity is an order of magnitude lower than that of graphite. Most of the nanotubes have metallic conductivity, and a smaller part exhibits the properties of a semiconductor with a band gap from 0.1 to 0.3 eV.
French and Russian researchers (from IPTM RAS, Chernogolovka) discovered another property of nanotubes, superconductivity. They measured the current-voltage characteristics of an individual single-walled nanotube with a diameter of ~1 nm, a large number of single-walled nanotubes rolled into a bundle, as well as individual multiwalled nanotubes. Superconducting current at temperatures close to 4K has been observed between two superconducting metal contacts. The features of charge transfer in a nanotube differ significantly from those inherent in ordinary, three-dimensional conductors and, apparently, are explained by the one-dimensional nature of the transfer.
Also discovered by de Geer from the University of Lausanne (Switzerland) interesting property: a sharp (about two orders of magnitude) change in conductivity with a small, 5-10°, bend of a single-walled nanotube. This property can expand the range of applications of nanotubes. On the one hand, the nanotube turns out to be a ready-made, highly sensitive converter of mechanical vibrations into an electrical signal and back (in fact, it is a telephone handset several microns long and about a nanometer in diameter), and, on the other hand, it is an almost ready-made sensor of the smallest deformations. Such a sensor could find application in devices that monitor the condition of mechanical components and parts on which the safety of people depends, for example, passengers of trains and airplanes, personnel of nuclear and thermal power plants, etc.
Capillary. Experiments have shown that an open nanotube has capillary properties. To open the nanotube, you need to remove the top part - the cap. One method of removal is to anneal the nanotubes at a temperature of 850 0C for several hours in the stream carbon dioxide. As a result of oxidation, about 10% of all nanotubes become open. Another way to destroy the closed ends of nanotubes is to soak them in concentrated nitric acid for 4.5 hours at a temperature of 2400 C. As a result of this treatment, 80% of the nanotubes become open.
The first studies of capillary phenomena showed that liquid penetrates into the nanotube channel if its surface tension is not higher than 200 mN/m. Therefore, to introduce any substances into nanotubes, solvents with low surface tension are used. For example, to introduce nanotubes of some metals into the channel, concentrated nitric acid is used, the surface tension of which is low (43 mN/m). Then annealing is carried out at 4000 C for 4 hours in a hydrogen atmosphere, which leads to the reduction of the metal. In this way, nanotubes containing nickel, cobalt and iron were obtained.
Along with metals, carbon nanotubes can be filled gaseous substances, for example hydrogen in molecular form. This ability has practical significance, because it opens up the possibility of safe storage of hydrogen, which can be used as an environmentally friendly fuel in engines internal combustion. Scientists were also able to place inside a nanotube a whole chain of fullerenes with gadolinium atoms already embedded in them (see Fig. 5).
Rice. 5. Inside C60 inside single-walled nanotube
Capillary effects and filling of nanotubes
nanotube carbon pyrolysis electric arc
Soon after the discovery of carbon nanotubes, the attention of researchers was attracted by the possibility of filling nanotubes various substances, which is not only of scientific interest, but also has great importance for applied problems, since a nanotube filled with a conducting, semiconducting or superconducting material can be considered the smallest of all microelectronic elements known to date. Scientific interest in this problem is associated with the possibility of obtaining an experimentally substantiated answer to the question: at what minimum sizes do capillary phenomena retain their features inherent in macroscopic objects? For the first time, this problem was considered in the problem of drawing a NR molecule inside nanotubes under the influence of polarization forces. It has been shown that capillary phenomena leading to the absorption of wetting liquids inner surface tubes inside the capillary retain their nature when moving to nanometer-diameter tubes.
Capillary phenomena in carbon nanotubes were first carried out experimentally in a study where the effect of capillary drawing of molten lead into the nanotubes was observed. In this experiment, an electric arc intended for the synthesis of nanotubes was ignited between electrodes with a diameter of 0.8 and a length of 15 cm at a voltage of 30 V and a current of 180 - 200 A. A layer of material 3-4 cm high formed on the cathode surface as a result of thermal destruction of the anode surface was removed from the chamber and kept for 5 hours at T = 850° C in a flow of carbon dioxide. This operation, which resulted in the sample losing about 10% of its mass, helped clear the sample of amorphous graphite particles and expose the nanotubes in the sediment. central part The sediment containing nanotubes was placed in ethanol and treated with ultrasound. The oxidation product dispersed in chloroform was applied to a carbon tape with holes for observation using an electron microscope. As observations showed, the tubes that were not subjected to treatment had a seamless structure, heads correct form and diameter from 0.8 to 10 nm. As a result of oxidation, about 10% of the nanotubes ended up with damaged caps, and some of the layers near the top were torn off. A sample containing nanotubes intended for observation was filled in a vacuum with drops of molten lead, which were obtained by irradiating a metal surface with an electron beam. In this case, lead droplets ranging in size from 1 to 15 nm were observed on the outer surface of the nanotubes. The nanotubes were annealed in air at T = 400°C (above the melting point of lead) for 30 minutes. As shown by the results of observations made using an electron microscope, part of the nanotubes after annealing turned out to be filled with solid material. A similar effect of filling nanotubes was observed when the heads of the tubes, which open as a result of annealing, were irradiated with a powerful electron beam. If the irradiation is strong enough, the material near the open end of the tube melts and penetrates. The presence of lead inside the tubes was determined by X-ray diffraction and electron spectroscopy. The diameter of the thinnest lead wire was 1.5 nm. According to the observation results, the number of filled nanotubes did not exceed 1%.
Tutoring
Need help studying a topic?
Our specialists will advise or provide tutoring services on topics that interest you.
Submit your application indicating the topic right now to find out about the possibility of obtaining a consultation.
Carbon nanotubes create new industry industry and materials science
Substances of the “nano” category, that is, with particles less than 100 nm, are today represented by carbon black (soot) and silica gel (“white soot”). The production volumes of other nanomaterials are incomparably lower. But now the situation is changing, carbon nanotubes have entered the market. Carbon nanotubes- these are extended cylindrical structures consisting of one or several hexagonal (geometrically similar to a honeycomb) graphite planes rolled into a tube
Carbon microtubes have been patented in late XIX centuries, and nanotubes were first obtained at the Moscow Institute of Physical Chemistry in the 1950s, then in Japan in the 1970s, and finally “discovered” in Japan in 1991. Since then, interest in pipes has grown steadily.
By set required properties nanotubes have no analogues
- The bond of carbon atoms with each other in nanotubes has a record strength. The Young's modulus (a pressure dimension that characterizes a substance's resistance to tension or compression) of nanotubes is more than 1 TPa (about 1 million atmospheres - higher than that of diamond). The thermal conductivity of nanotubes is eight times higher than that of copper, and the electrical conductivity does not obey Ohm's law. The current density in the tubes can be a thousand times higher than the density at which copper wire explodes.
Global production of nanotubes has exceeded 1,000 tons per year. The use of materials made from carbon nanotubes or containing carbon nanotubes has become a new economic sector that was not affected by the global financial crisis.
- The global demand for nanotubes in 2010 is estimated at 10 thousand tons. They are produced by more than 40 companies. German Bayer plans to expand production capacity to 3,000 t/y by 2012, the French Arkema has a plant with an annual capacity of 400 tons, Chinese CNano - 500 t/y, and Belgian Nanocyl - 400 t/year. Japanese company increases production of carbon nanofibers up to 500 t/y Showa Denko .
- Nanostructured materials are divided into two large groups. The materials of one consist of 95–100% nanotubes. The second materials are nanocomposites - on the contrary, they contain little nanotubes, up to 5%.
Nanotube materials
The shape of nanotubes allows them to be arranged in two ways: chaotically or orderedly, which affects the properties of materials. Nanotubes can be modified by attaching various chemical groups and nanoparticles. This also changes the properties of the nanotubes themselves and their materials.
- The materials of the first group include “monolithic” structures made of nanotubes; coatings, films and nanopapers from tubes; fibers from tubes; “forest” - nanotubes located parallel to each other and perpendicular to the substrate. “Monolithic” materials are not widely used.
“Rubber” has been isolated from tangled long nanotubes, resistant to destruction under cyclic loads and temperatures from –140 to +900 °C. Its performance is far superior to silicone rubber, which is considered the best viscoelastic material.
- Coatings, films and nanopapers are obtained either during the synthesis of tubes or from their dispersions (colloidal solutions). The first group of methods is high-temperature, the second does not require heating. The simplest macromaterial from tubes, nanopaper, has a thickness of 10–30 nm and is produced by filtration of dispersions.
 .
.
Company Nanocomp Technologies (USA) sells sheets of nanopaper with an area of about 3 m2 and plans to create a production facility with a capacity of 4–6 t/year. Methods for producing nanopaper rolls have been implemented.
- Filters are made from nanopaper (including for removing viruses or desalting water), protection against electromagnetic radiation, heater parts, sensors, actuators, field emitters, electrodes of electrochemical devices, catalyst carriers, etc.
Transparent electrically conductive films and coatings compete with solid solutions of indium and tin oxides and can replace this expensive and fragile material in electronics, sensors and photovoltaics devices.
- American company Eikos developed and has been supplying the composition since 2005 Invisicon ink for deposition of thin films of nanotubes onto substrates.
Carbon nanotube fibers seemed like an ideal “space elevator” tether material for economically lifting cargo into Earth orbit. However, transferring the properties of nanotubes to macromaterials turned out to be far from a simple task.
- Fibers are obtained in different ways. “Dry” methods include formation from airgel formed during the pyrolysis of hydrocarbons and spinning from “wood”.
The technology of pulling and twisting fibers from airgel - “soft smoke” - was developed in Cambridge University . Into the reaction zone with high temperature hydrocarbon is supplied, from which an airgel is formed (i.e., a gel in which the liquid phase is completely replaced by a gaseous one). Fiber is spun from it, like in the old days from tow. In Israel, a company was created in 2010 to produce body armor and protective coatings from hybrid composites containing Cambridge nanotubes.
- Spinning from the “forest” is reminiscent of obtaining silk threads from silkworm cocoons.
 .
.
Solution methods for producing fibers are extrusion of dispersions into a liquid stream or extraction from colloidal solutions in superacids (acids stronger than sulfuric).
- Company Nanocomp Technologies announced the supply of strong fibers up to 10 km long, for the production of which long nanotubes are used. Twisted threads have a strength of 3 GPa and in some respects are already superior to Kevlar.
“Forest” has no analogues in its set of properties - it is an elastic, electrically and thermally conductive material that can take different shapes and be modified. In 2004, a high-throughput “forest” supergrowth process was described: producing very pure carbon nanotubes up to 15–18 mm long, which significantly reduces their cost.
- Japan is preparing to launch production based on the supergrowth process. Its capacity is only 600 g/h of single-walled nanotubes, but they plan to soon increase it to 10 t/g.
"Forest" can be used to create electrodes for supercapacitors, field emitters and solar panels, as a component of polymer-based composites. By laying the “scaffold” on the surface of the substrate, dense ribbons were obtained. They can surpass metals in electrical conductivity and will find application in the aerospace industry.
- Artificial muscle tapes made from parallel nanotubes operate at temperatures from 80 to 1900 K and when applied electric potential provide very high elongation. Such converters of electricity into mechanical energy are much more efficient than piezocrystals.
Materials doped with nanotubes
The production of materials of the second group - nanocomposites, mainly polymers - is growing sharply
- The introduction of even small amounts of carbon nanotubes noticeably changes the properties of polymers, imparts electrical conductivity, increases thermal conductivity, and improves mechanical characteristics, chemical and thermal stability. Nanocomposites based on dozens of different polymers have been created, and many methods for their preparation have been developed.
Composite fibers based on polymers with nanotubes can find wide application.
- Almost everything produced by the company Bayer Nanotubes are used for polymer composites. Company Arkema supplies its nanotubes for thermoplastic composites, and Nanocyl - for heat-shrinkable polymers and prepregs with carbon fibers (prepregs are semi-finished composite materials for further processing).
American company Hyperion Catalysis Int. , a pioneer in the industrial production of nanotubes, produces concentrates for incorporation into epoxy resins and polymers.
 Types of nanotubes
Types of nanotubes
- Ceramic composites are created on the basis of many refractory substances, but in terms of industrial development they are noticeably inferior to nanocomposites based on polymers. As in the case of polymers, the addition of small amounts of nanotubes increases electrical and thermal conductivity, imparts the ability to protect against electromagnetic radiation, and most importantly, increases the crack resistance of ceramics.
The introduction of very small amounts of nanotubes into concrete increases its grade, crack resistance, strength and reduces shrinkage.
- Metal composites are created with common non-ferrous metals and alloys. Most attention is given to copper composites, the mechanical properties of which are two to three times higher than those of copper. Many compositions have increased strength and hardness, lower coefficients of thermal expansion and friction.
Hybrid composites typically contain three components: polymer or inorganic fibers (fabrics), nanotubes and a binder. This class includes prepregs .
- Specializes in the production of prepregs with nanotubes American company Zyvex Performace Materials . Nanotubes increase the strength and stiffness of prepregs by 30–50%. Prepregs are used to create unmanned maritime reconnaissance boats "Piranha" .
In 2009, the first aerial acrobatics aircraft with an engine fairing made of a composite with nanotubes flew in the United States. Some elements of an airplane glider F-35 companies Martin Lockheed made from such composites, approximately 100 parts of the passenger airframe Boeing 787 supposed to be done using nanotubes.
- Company Nanocyl produces epoxy resin with tubes Epocyl and prepregs Pregcyl based on glass fibers, carbon or aramid fibers. Additives increase crack resistance by 100%, interlayer shear strength by 15% and reduce the coefficient of thermal expansion. It is planned to use composites in the automotive and aviation industries, for body armor. They reduce the weight of 49-meter wind turbine blades from 7.3 to 5.8 tons.
Finnish company Amroy Europe Oy using nanotube production Bayer , produces epoxy concentrate Hybtonite For sea vessels, wind generators, sports equipment, etc.
- For prepregs Canadian Nanoledge uses company tubes Bayer , A Nanocomp Technologies produces large sheets and rolls of nanopaper.
Hybrid composites can exhibit damage sensor properties.
- Biocomposites have also been created with various matrices. Materials for bone implants, films for growing muscle and bone tissue, retina and epithelial cells of the eye, networks of neurons, as well as biofunctional composites and biosensors are being studied.

The examples do not exhaust the diversity and properties of materials with nanotubes. Their areas of application are expanding; they are beginning to determine the level of development of nanostructured materials science and the general state of science and technology in individual countries.
Eduard Rakov, Doctor of Chemical Sciences, Head of the Department of Nanotechnology and Nanomaterials, Russian Chemical Technical University named after. DI. Mendeleev
Introduction:
Nanotubes can act not only as a material under study, but also as a research tool. Based on nanotubes, for example, it is possible to create microscopic scales. We take a nanotube, determine (by spectroscopic methods) the frequency of its natural vibrations, then attach the sample under study to it and determine the frequency of vibrations of the loaded nanotube. This frequency will be less than the oscillation frequency of a free nanotube: after all, the mass of the system has increased, but the rigidity has remained the same (remember the formula for the oscillation frequency of a weight on a spring). For example, in the work it was discovered that the load reduces the oscillation frequency from 3.28 MHz to 968 kHz, from where the weight of the load was obtained 22 +- 8 fg (femtograms, i.e. 10-15 grams!)
Another example where a nanotube is part of a physical device is to “mount” it on the tip of a scanning tunneling or atomic force microscope. Usually such an edge is a sharpened tungsten needle, but by atomic standards such sharpening is still quite rough. A nanotube is an ideal needle with a diameter of the order of several atoms. By applying a certain voltage, it is possible to pick up atoms and entire molecules located on the substrate directly under the needle and transfer them from place to place.
The unusual electrical properties of nanotubes will make them one of the main materials for nanoelectronics. Prototypes of field-effect transistors based on a single nanotube have already been created: by applying a blocking voltage of several volts, scientists have learned to change the conductivity of single-walled nanotubes by 5 orders of magnitude!
Several applications of nanotubes in the computer industry have already been developed. For example, prototypes of thin flat displays operating on a matrix of nanotubes have been created and tested. Under the influence of a voltage applied to one end of the nanotube, electrons begin to be emitted from the other end, which fall on the phosphorescent screen and cause the pixel to glow. The resulting image grain will be fantastically small: on the order of a micron!
Carbon nanotubes (tubulenes) are extended cylindrical structures with a diameter from one to several tens of nanometers and a length of up to several centimeters, consisting of one or several hexagonal graphite planes rolled into a tube and usually ending in a hemispherical head, which can be considered as half a fullerene molecule
Nanotube structure:
To obtain a nanotube (n, m), the graphite plane must be cut along the directions of the dotted lines and rolled along the direction of the vector R .
An ideal nanotube is a graphite plane rolled into a cylinder, that is, a surface lined with regular hexagons with carbon atoms at the vertices. The result of such an operation depends on the angle of orientation of the graphite plane relative to the axis of the nanotube. The orientation angle, in turn, determines the chirality of the nanotube, which determines, in particular, its electrical characteristics
The chirality of nanotubes is indicated by a set of symbols (m, n) indicating the coordinates of a hexagon, which, as a result of folding the plane, must coincide with the hexagon located at the origin.
Another way to indicate chirality is to indicate the angle α between the direction of folding of the nanotube and the direction in which neighboring hexagons have common side. However, in this case for full description The geometry of the nanotube must indicate its diameter. The chirality indices of a single-walled nanotube (m, n) uniquely determine its diameter D. The indicated relationship has the following form:
Where d 0 = 0.142 nm - the distance between neighboring carbon atoms in the graphite plane. The relationship between chirality indices (m, n) and angle α is given by the relation:
Among the various possible directions of folding of nanotubes, those for which alignment of the hexagon (m, n) with the origin of coordinates does not require distortion of its structure are distinguished. These directions correspond, in particular, to the angles α = 0 (armchair configuration) and α = 30° (zigzag configuration). The indicated configurations correspond to chiralities (m, 0) and (2n, n), respectively.
 (types of nanotubes)
(types of nanotubes) Single-walled nanotubes:
The structure of single-walled nanotubes observed experimentally differs in many respects from the idealized picture presented above. First of all, this concerns the vertices of the nanotube, the shape of which, as follows from observations, is far from an ideal hemisphere.
A special place among single-walled nanotubes is occupied by the so-called armchair nanotubes or nanotubes with chirality (10, 10). In nanotubes of this type, two of the C-C bonds that make up each six-membered ring are oriented parallel to the longitudinal axis of the tube. Nanotubes with a similar structure should have a purely metallic structure.
Multi-walled nanotubes:
Multi-walled nanotubes differ from single-walled nanotubes in a much wider variety of shapes and configurations. The variety of structures is manifested in both longitudinal and transverse directions.
The “Russian dolls” type structure (Fig. a) is a collection of cylindrical tubes coaxially nested into each other. Another type of this structure (Fig. b) is a collection of coaxial prisms nested within each other. Finally, the last of the above structures (Fig. c) resembles a scroll. For all structures in Fig. The characteristic value of the distance between adjacent graphite layers is close to the value of 0.34 nm, inherent in the distance between adjacent planes of crystalline graphite.
The implementation of a particular structure of multi-walled nanotubes in a specific experimental situation depends on the synthesis conditions. Analysis of available experimental data indicates that the most typical structure Multi-walled nanotubes are a structure with sections of the “Russian nesting doll” and “papier-mâché” type alternately located along the length. In this case, smaller “tubes” are sequentially inserted into larger tubes. This model is supported, for example, by facts on the intercalation of potassium or ferric chloride into the “intertubular” space and the formation of “bead”-type structures.
Discovery history:
As is known, fullerene (C 60) was discovered by the group of Smalley, Kroto and Curl in 1985, for which in 1996 these researchers were awarded Nobel Prize in chemistry. As for carbon nanotubes, it cannot be called the exact date their discoveries. Although Iijima's observation of the structure of multi-walled nanotubes in 1991 is well known, there is earlier evidence for the discovery of carbon nanotubes. So, for example, in 1974-1975. Endo et al. have published a number of papers describing thin tubes with a diameter less than 100 Å prepared by vapor condensation, but a more detailed structural study has not been carried out. In 1977, a group of scientists from the Institute of Catalysis of the Siberian Branch of the USSR Academy of Sciences, while studying the carbonization of iron-chromium dehydrogenation catalysts under a microscope, recorded the formation of “hollow carbon dendrites”; a mechanism of formation was proposed and the structure of the walls was described. In 1992, an article was published in Nature, which stated that nanotubes were observed in 1953. A year earlier, in 1952, an article by Soviet scientists Radushkevich and Lukyanovich reported electron microscopic observation of fibers with a diameter of about 100 nm, obtained from the thermal decomposition of oxide carbon on an iron catalyst. These studies were also not continued.